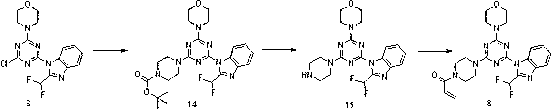Piperazinotriazines as PI3K inhibitors for use in the treatment antiproliferative disorders
A compound, ethyl technology, applied in the field of piperazino triazine compounds used as PI3K inhibitors for the treatment of anti-proliferative disorders, which can solve the problems of control mechanism failure and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
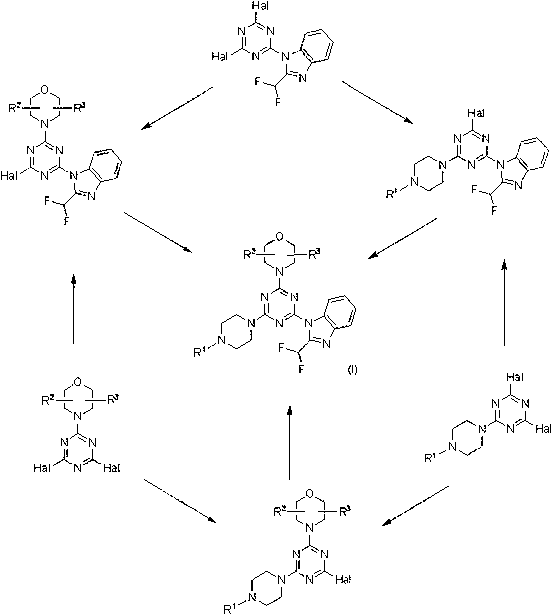
[0100] Compounds were synthesized according to the procedure in Scheme 2 5-7 , 11 and 12 :
[0101] Process 2
[0102]
[0103] Substitute morpholine for Cyanuric chloride in dichloromethane at -50°C 1 After 20 min, an intermediate was generated 2 . K in DMF at -5°C 2 CO 3 In the presence of , the second chloride was replaced by 2-difluoromethyl-1H-benzimidazole for 30 min, and further stirred at room temperature for 4 h to generate the intermediate 3 . at room temperature through the K 2 CO 3 and the presence of DMF to make the intermediate 3 After 45 minutes of amination, the final step yields the product 4-7、11 and 12 . compound 4 is a known compound ZSTK474 prepared for comparison purposes.
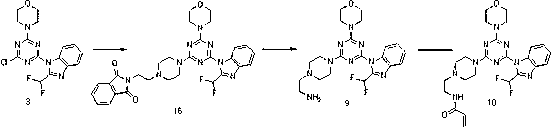
[0104] Amination with BOC-protected piperazine, followed by BOC deprotection and reaction with acrylic anhydride, from intermediate 3 get compound 8 . The phthalimide protecting group is cleaved by amination with 2-(2-(piperazin-1-yl)ethyl)isoindoline-1,3-dione...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com