Method for simultaneous production of methyl ethyl ketone and cyclohexane
A technology of methyl ethyl ketone and cyclohexane, which is applied in the field of simultaneous production of methyl ethyl ketone and cyclohexane, can solve the problems of high construction cost, large floor area, and large production investment, and achieve high utilization efficiency and no potential safety hazards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: preparation catalyst
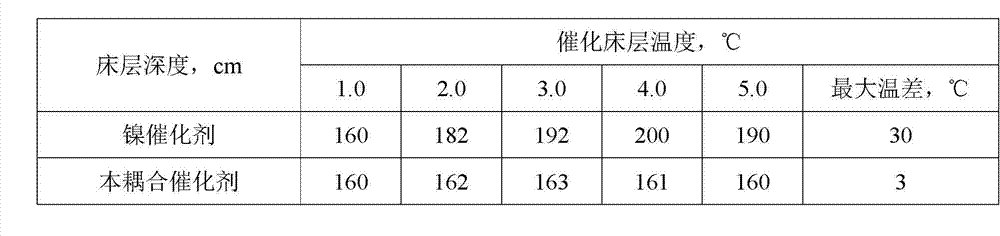
[0035] Weigh Cu(NO 3 ) 2 ﹒ 3H 2 O, Ni(NO 3 ) 2 ﹒ 6H 2 O, Zn(NO 3 ) 2 ﹒ 6H 2 O and Al(NO 3 ) 3 ﹒ 9H 2 O and SiO 2 Choose at least one of the two to dissolve in water to form solution I with a concentration of 200~300g / L, and weigh Na 2 CO 3 Dissolve in water to make solution II with a concentration of 70~100g / L, add solution I dropwise to solution II at 50°C for 12 minutes, and control the final pH value to 7.0. After the dropwise addition, aged at 60° C. for 0.5 h, filtered, washed, and dried at 100° C. for 8 h to obtain a catalyst precursor. The precursor was calcined in a muffle furnace at 340°C for 4 hours in an air atmosphere, and pressed into pellets to obtain the coupled catalyst.
[0036] Cu(NO 3 ) 2 ﹒ 3H 2 O, Ni(NO 3 ) 2 ﹒ 6H 2 O, Zn(NO 3 ) 2 ﹒ 6H 2 O, Al(NO 3 ) 3 ﹒ 9H 2 O and SiO 2 The dosage ratio of at least one of the two is as follows:
[0037] (1) Ni(NO 3 ) 2 ﹒ 6H 2 O and Cu(NO ...
Embodiment 2
[0080] Accurately measure the close-packed coupling catalyst (the composition of the first-stage catalyst: the molar ratio of nickel to copper is 1:2, the molar ratio of copper to zinc is 1.5:1, alumina is the carrier, and the mass percentage of the carrier is 13.0%; the composition of the second-stage catalyst is : The molar ratio of nickel to copper is 2:1, the molar ratio of copper to zinc is 1.5:1, alumina is the carrier, and the mass percentage of the carrier is 13.0%) 30mL are respectively loaded into two-stage catalytic reactors (the first stage adopts tube heat exchange type reactor, the second stage uses an adiabatic reactor), after the catalyst is filled, carry out an airtight test, use soapy water to check for leaks until no bubbles come out, and use 5% H 2 -95%N 2 (Volume percent) The mixed gas is activated by temperature programming (reducing nickel and copper oxides to zero-valent or close to zero-valent nickel and copper), programmed temperature program: 15°C, 2...
Embodiment 3
[0082] Accurately measure the close-packed coupling catalyst respectively (the composition of the first-stage catalyst: the molar ratio of nickel to copper is 1:8, the molar ratio of copper to zinc is 3:1, alumina is the carrier, and the mass percentage of the carrier is 15.0%; the composition of the second-stage catalyst is : The molar ratio of nickel to copper is 5:1, the molar ratio of copper to zinc is 3.0:1, alumina is the carrier, and the mass percentage of the carrier is 15.0%) 30mL are respectively loaded into two-stage catalytic reactors (the first stage uses tube heat exchange type reactor, the second stage uses an adiabatic reactor), after the catalyst is filled, carry out an airtight test, use soapy water to check for leaks until no bubbles come out, and use 5% H 2 -95%N 2 (Volume percent) The mixed gas is activated by temperature programming (reducing nickel and copper oxides to zero-valent or close to zero-valent nickel and copper), programmed temperature program...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com