Methoxy polyethylene glycol-polyphosphate diblock copolymer and adriamycin bonding medicine thereof
A technology of polyethylene glycol monomethyl ether and polyphosphate doxorubicin, which is applied in drug combinations, antineoplastic drugs, and pharmaceutical formulations, can solve problems such as limited side group selection, side reactions, and interference with polymerization reactions, and achieve Simple preparation method, good stability and high reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1, mPEG a -PAOOP b Synthesis and characterization of
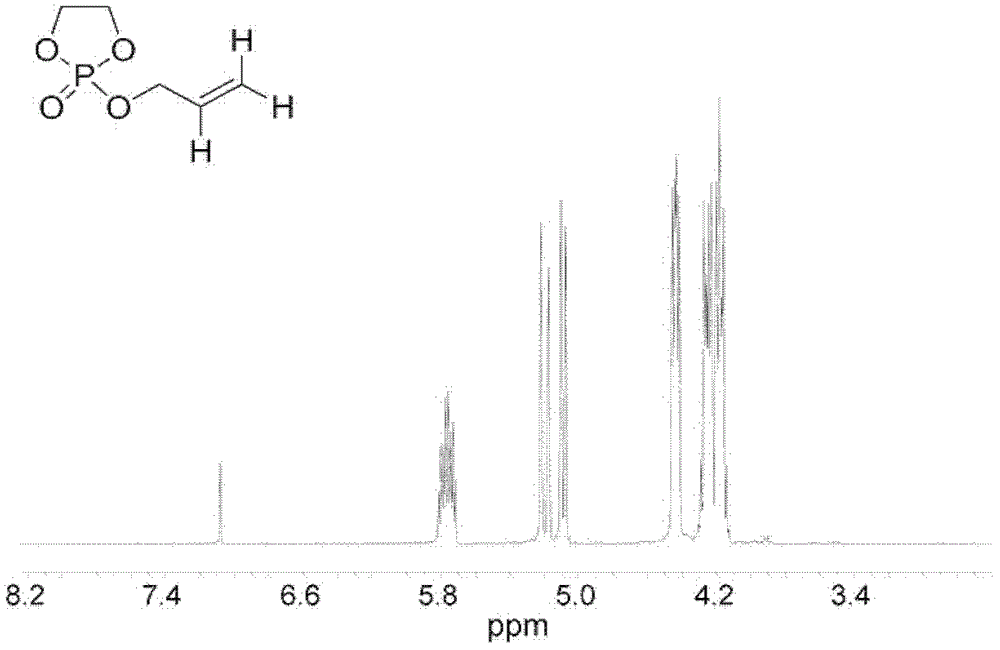
[0069] 1. Synthesis and characterization of 2-allyloxy-2-oxo-1,3,2-dioxaphospholane (AOOP) cyclic phosphate monomer
[0070] (1) Synthesis of 2-chloro-2-oxo-1,3,2-dioxaphospholane (COP)
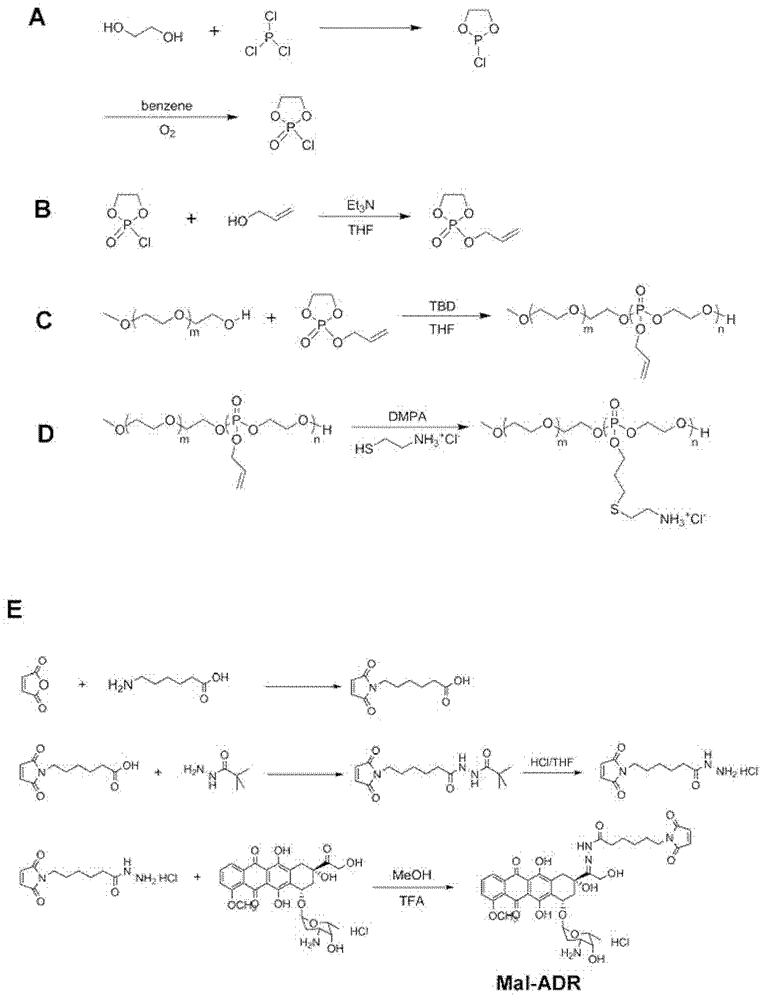
[0071] COP synthesis route such as figure 1 (A) shown. The specific steps for synthesizing COP are as follows: at 25°C, slowly add 301 mL of 3.25 mol / L ethylene glycol in dichloromethane to 300 mL of 3.26 mol / L phosphorus trichloride in dichloromethane. After the dropwise addition was completed, the reaction was continued for 0.5 hour, and the solvent was distilled off under normal pressure at 45°C. After obtaining the product twice in a row under reduced pressure distillation (20Pa), the product was dissolved in 500mL of benzene, and logically 3 days. 2 Until the reaction is complete, distillation under reduced pressure (20Pa) is performed again, and the fraction at 70°C is collected to obtain COP.
[0072] (2) S...
Embodiment 2
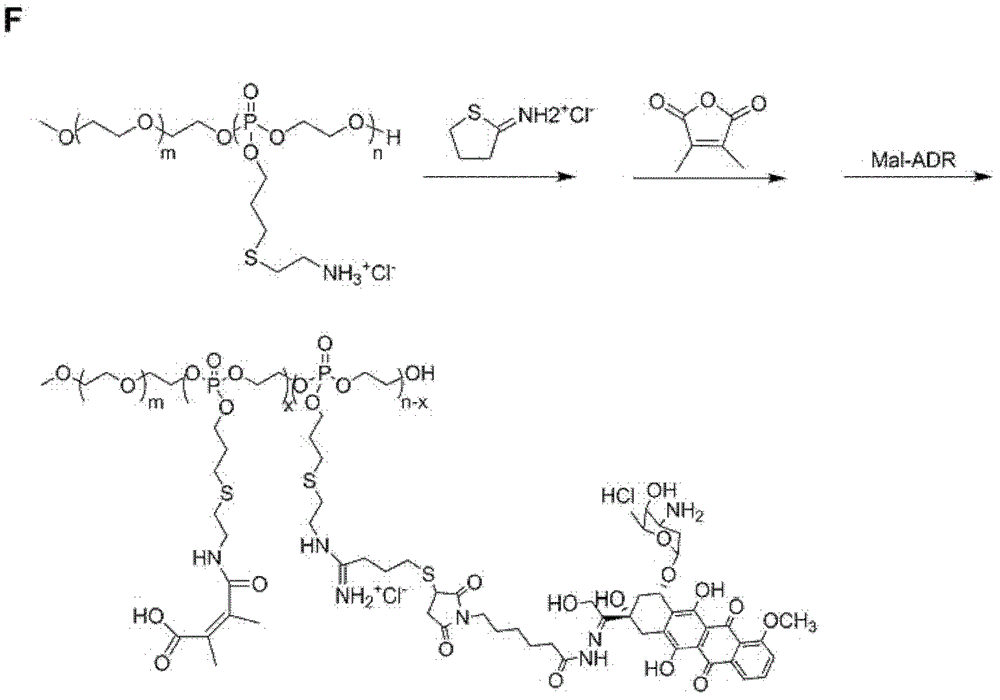
[0092] Embodiment 2, the synthesis and the characterization (mPEG a -PAOOP b -Cya-ADR-DMMA)
[0093] 1. Polyethylene glycol monomethyl ether-polyphosphate diblock copolymer (mPEG) with amino groups as side groups a -PAOOP b -Cya) synthesis and characterization
[0094] Synthetic route such as figure 1 (D) as shown in mPEG4 5 -b-PAOOP 75 As an example, at 25°C, take mPEG 45 -b-PAOOP 75 (200mg, 0.014mmol), mercaptoethylamine hydrochloride (0.375g, 3.15mmol, 300% of the double bond molar weight) and benzoin dimethyl ether (DMPA, 13.4mg, 0.05mmol, 5% of the double bond molar weight) Dissolve in 1.5 mL dry DMF. After bubbling nitrogen gas for 20 minutes, irradiate with 365nm ultraviolet light for 30 minutes, dialyze the product with distilled water for 48 hours at 4°C (molecular weight cut-off is 2000), and obtain polyethylene glycol monomethyl ether-polyphosphoric acid with side groups as amino groups after freeze-drying Ester polymer (mPEG 45 -PAOOP 75 -Cya, PPC). Pr...
Embodiment 3
[0101] Embodiment 3, mPEG a -PAOOP b -Cya-DMMA and mPEG a -PAOOP b -Biocompatibility of Cya-ADR-DMMA copolymer nanoparticles
[0102] 1. mPEG a -PAOOP b -Biocompatibility of Cya-DMMA nanoparticles
[0103] In this experiment, since ADR itself has the ability to kill cells, in order to eliminate the interference of ADR on the biocompatibility of the material, mPEG without doxorubicin bound to the side group was selected. a -PAOOP b -Cya-DMMA. The above materials were prepared as a 1 mg / mL nanoparticle solution and then diluted to 10.6-340 μg / mL. Determination of different concentrations of mPEG by MTT method a -PAOOP b - Cytotoxicity of Cya-DMMA copolymer nanoparticles. The specific test is: different concentrations of mPEG a -PAOOP b - After the Cya-DMMA nanoparticle solution was co-cultured with the MDA-MB-231 cells for 48 hours under the conditions of pH=7.4 and pH=6.8, respectively, the cell viability was measured. The survival rate of cells under different tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com