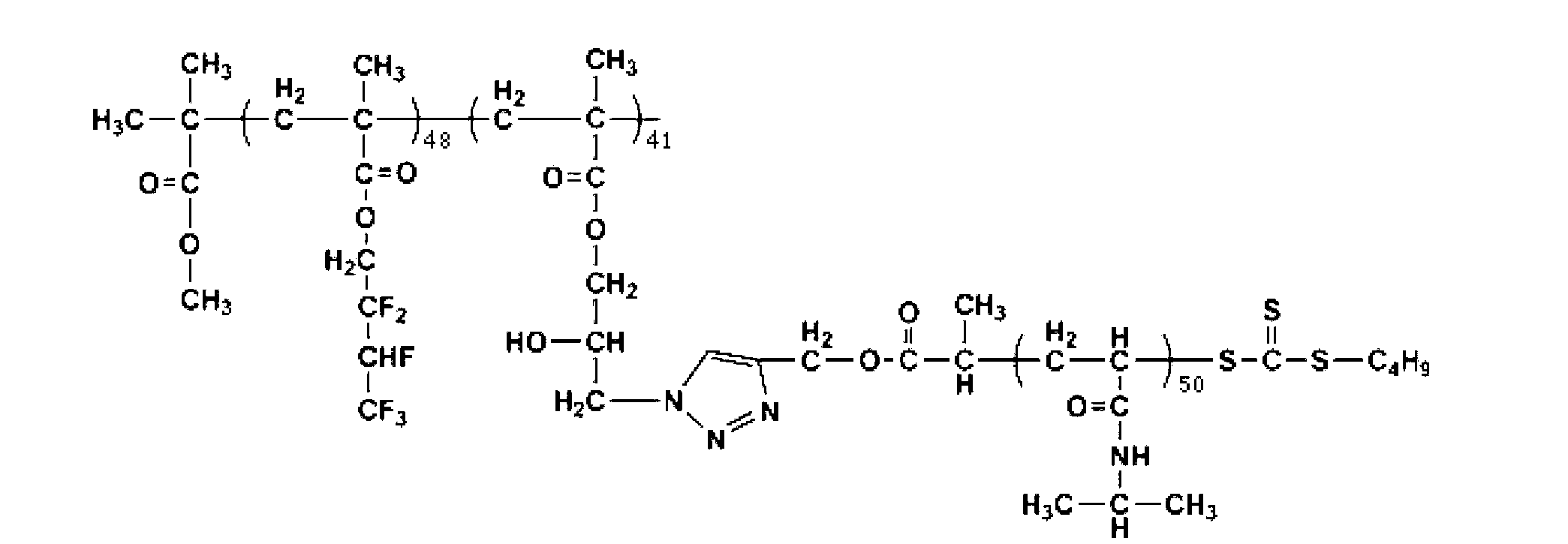
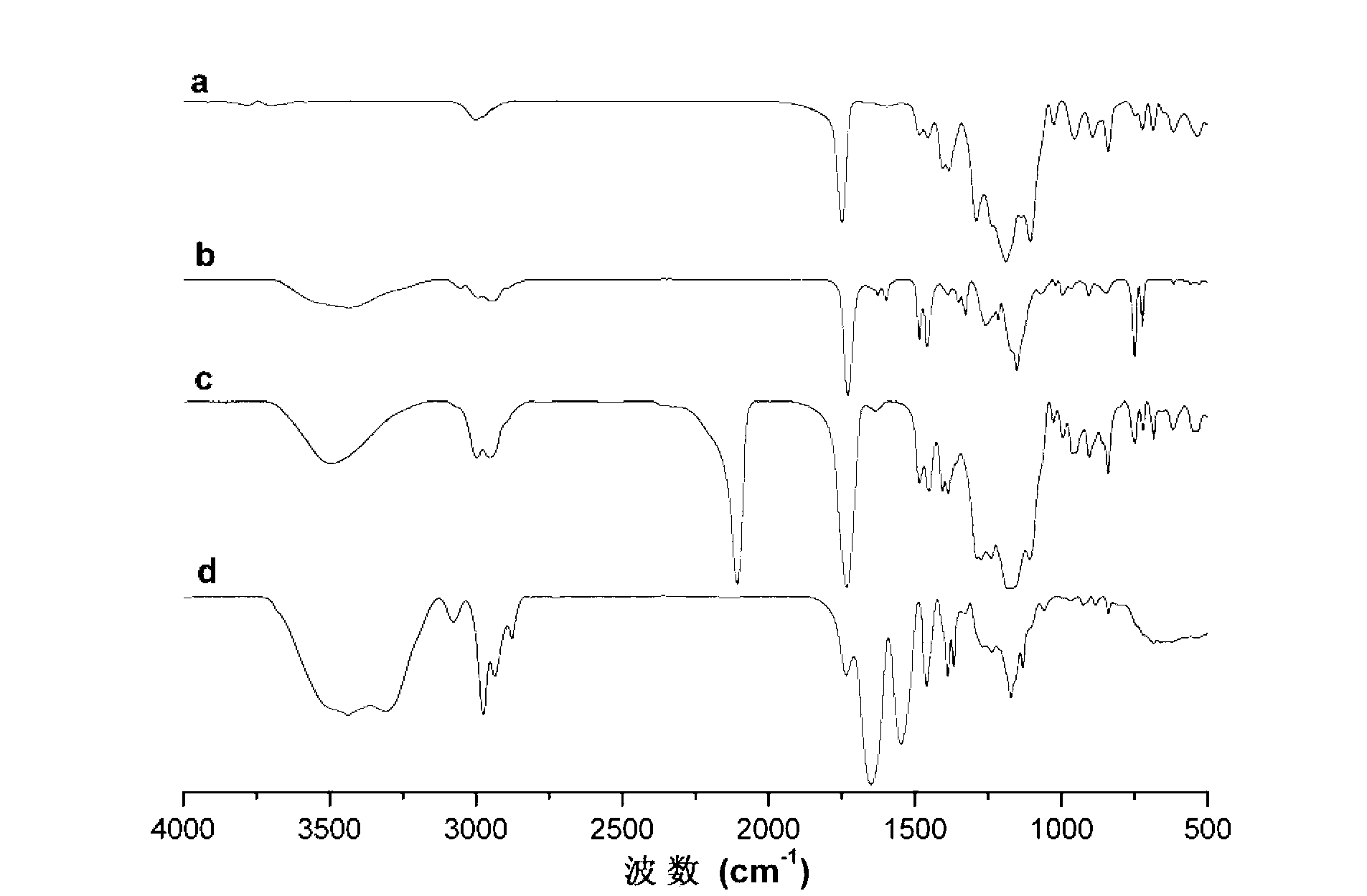
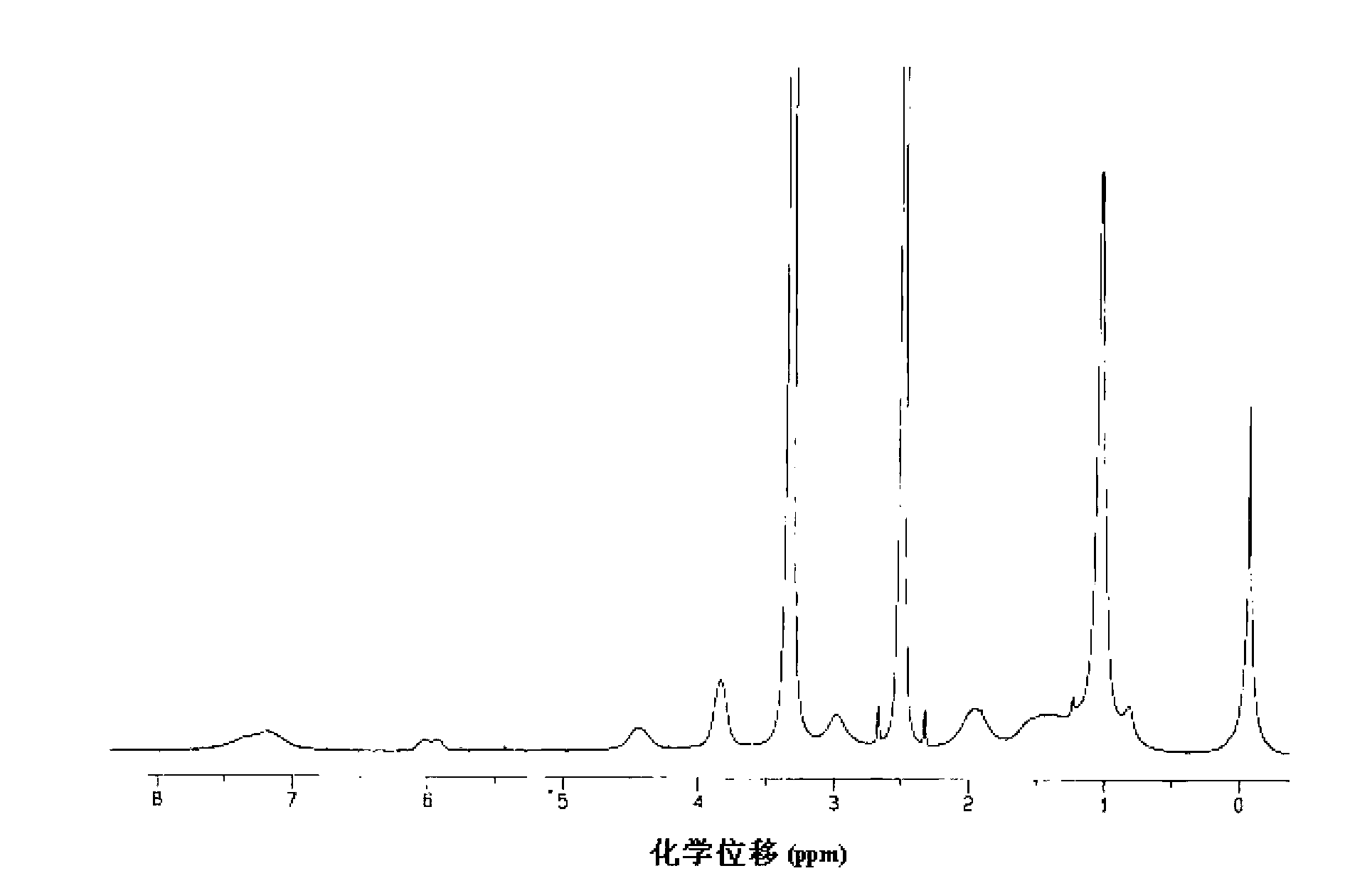
Fluorine-containing block graft polymer with thermosensitivity and preparation method and application thereof
A graft polymer, temperature-sensitive technology, applied in the field of polymer material preparation, can solve the problems of increased steric hindrance, difficult operation, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: PHFBMA-b-(PGMA-g-PNIPAM) and its preparation
[0057] (1) Preparation of PHFBMA: 14.32 g of hexafluorobutyl methacrylate (HFBMA), 0.19 g of initiator methyl 2-bromoisobutyrate, 0.17 g of cuprous bromide, 0.05 g of bromide Add copper and 15mL anisole to a 50mL single-mouth reaction bottle and seal the bottle mouth. After bubbling argon gas-freezing-thawing three cycles for three times, add 0.52g bipyridine and continue through three cycles once, and place at 65 React in an oil bath at ℃ for 6 hours, freeze with liquid nitrogen and dilute with dichloromethane, stir and oxidize for 1 hour, remove the catalyst through neutral alumina, concentrate at room temperature for 30 minutes, precipitate in 150 mL of n-hexane, filter and evaporate the solvent, and take the residue for use After dissolving 7mL of tetrahydrofuran and 200mL of n-hexane for precipitation and cycling twice, the precipitate was collected and vacuum-dried at room temperature to obtain 9.35g of flu...
Embodiment 2
[0073] Example 2: PHFBMA-b-(PGMA-g-PDNIPAM) and its preparation
[0074] (1) Preparation of PHFBMA: same as step (1) of Example 1;
[0075] Preparation of PHFBMA-b-PGMA copolymer: same as step (1) of Example 1;
[0076] (2) Preparation of PDNIPAM: 7.46g of temperature-sensitive monomer N,N-diisopropylacrylamide, 0.28g of chain transfer agent S-dodecyl-S'-(α-methyl-α "-propargyl isopropionate) trisulfide, 0.014g initiator AIBN and 14mL of purified dioxane were all added to a 25mL single-necked flask filled with argon and sealed well, after gas blowing-freezing- After three cycles of thawing, put it in an oil bath at 75°C for 7 hours, then stop the reaction in an ice-water bath, distill off most of the dioxane under reduced pressure at 45°C and precipitate in 200mL of cold ether, take the precipitate, and dry it at 25°C Dissolve in 4mL tetrahydrofuran and precipitate in 200mL cold diethyl ether (repeat this operation 3 times), filter, take the precipitate and dry it in vacuum ...
Embodiment 3
[0086] Example 3: PHFBMA-b-(PGMA-g-PDMAEMA) and its preparation
[0087] (1) Preparation of PHFBMA: same as step (1) of Example 1;
[0088] Preparation of PHFBMA-b-PGMA copolymer: same as step (1) of Example 1;
[0089] (2) Preparation of PDMAEMA: 8.76g of temperature-sensitive monomer dimethylaminoethyl methacrylate, 0.25g of chain transfer agent S-dodecyl-S'-(α-methyl-α" -propargyl isopropionate) trisulfide, 0.018g initiator AIBN and 25mL dioxane were added to a 50mL single-necked flask filled with argon and sealed, and after three cycles of blowing-freezing-thawing, placed in After reacting in an oil bath at 65°C for 6 hours, place it in an ice-water bath to stop the reaction. Distill under reduced pressure at 46°C to remove most of the dioxane and precipitate in 200 mL of cold ether. Take the precipitate, dry it at 25°C and dissolve it in 4 mL of tetrahydrofuran. Finally, precipitate in 200mL of cold ether (repeat this operation 3 times), filter, take the precipitate and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com