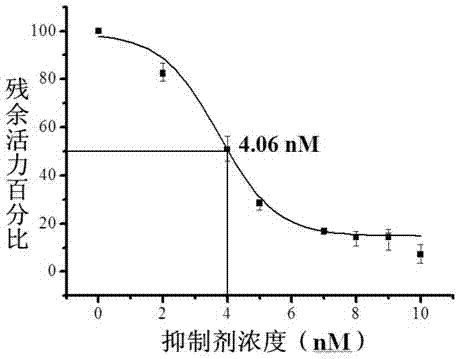
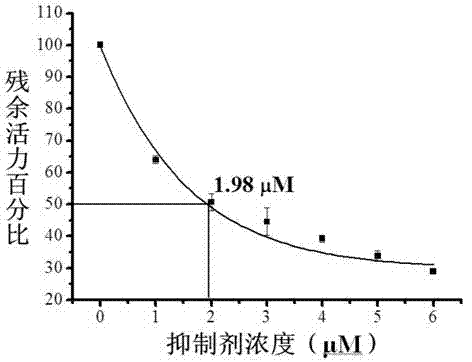
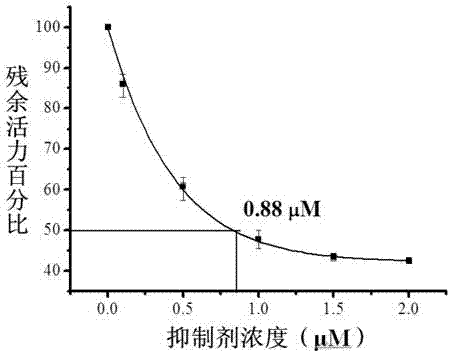
Hydrazine cathepsin K inhibitor and application thereof in treating osteoporosis
A technology for cathepsin and osteoporosis, applied in the field of cathepsin inhibitors, can solve the problems of complex action of cathepsin K and unsatisfactory selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067]
[0068] In a 100mL round bottom flask, add 0.61g E4, 0.44g phenylboronic acid, 0.0653g Pd(dppf)Cl 2 and 0.5g K 2 CO 3 , use 35mL of THF as the reaction solvent, add 2mL of water, after three filling and three pumping, reflux. After 6h, evaporate the THF in the system to dryness with a rotary evaporator, add ethyl acetate to dissolve the residue, successively water (once), saturated NaHCO 3 (2 times), water (1 time), and saturated NaCl (1 time) washed the organic phase successively. Finally with anhydrous Na2 SO 4 Dry, evaporate to dryness, and then use THF:PE=1:8 system as developing solvent to carry out silica gel column chromatography separation and purification to obtain white powdery solid E5. The characterization results are as follows:
[0069] 1 H NMR (500MHz, CDCl 3 ): δ7.86(d, J=5.6Hz, 2H), 7.64(dt, J=8.6, 6.1Hz, 4H), 7.46(t, J=7.6Hz, 2H), 7.39(t, J=7.3Hz , 1H), 6.64(d, J=8.3Hz, 1H), 5.34(td, J=10.1, 3.7Hz, 1H), 3.36(s, 3H), 3.24(s, 3H), 1.82(dd, J=...
Embodiment 2
[0072]
[0073] Put 0.41g of pyridine boronic acid and 0.39g of pinacol in a 50mL round bottom flask, add an appropriate amount of toluene as a solvent, and reflux. During the whole process, the evaporated water is continuously released from the system. Stop the reaction after 4 h, and check whether the reaction is complete by thin layer chromatography (TLC). The round-bottomed flask was cooled at room temperature, and suction filtered to obtain 0.67 g of pyridine borate as a white solid. Take 0.6g of pyridine borate, and other reaction materials, conditions and post-treatment process are the same as the synthesis route of E5. Finally, E6 was obtained as a crystalline solid. The characterization results are as follows:
[0074] 1 H NMR (500MHz, CDCl 3 ): δ8.67(d, J=4.3Hz, 2H).7.64(d, J=8.1Hz, 2H), 7.53-7.40(m, 4H), δ4.23(t, J=7.4Hz, 1H) , 3.20(s, 4H), 2.58(s, 2H), δ2.10(m, 1H), 1.55(ddd, J=20.1, 17.8, 11.1Hz, 2H), 1.01-0.96(m, 6H).
[0075] MS (ESI) m / z: [M+H] + : 3...
Embodiment 3
[0077]
[0078] Take 0.46g of thiophene boronic acid, and other reaction raw materials, conditions and post-treatment process are the same as the synthesis route of E5. Finally, E7 was obtained as a crystalline solid. The characterization results are as follows:
[0079] 1 H NMR (500MHz, DMSO): δ7.64(d, J=7.7Hz, 1H), 7.52(dd, J=19.7, 4.1Hz, 1H), 7.36(t, J=7.4Hz, 1H), 7.14( dd, J=5.0, 3.7Hz, 1H), δ4.23(t, J=7.4Hz, 1H), 3.20(s, 4H), 2.58(s, 2H), δ2.10(m, 1H), 1.55 (ddd, J=20.1, 17.8, 11.1Hz, 2H), 1.01-0.96(m, 6H).
[0080] MS (ESI) m / z: [M+H] + : 342.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com