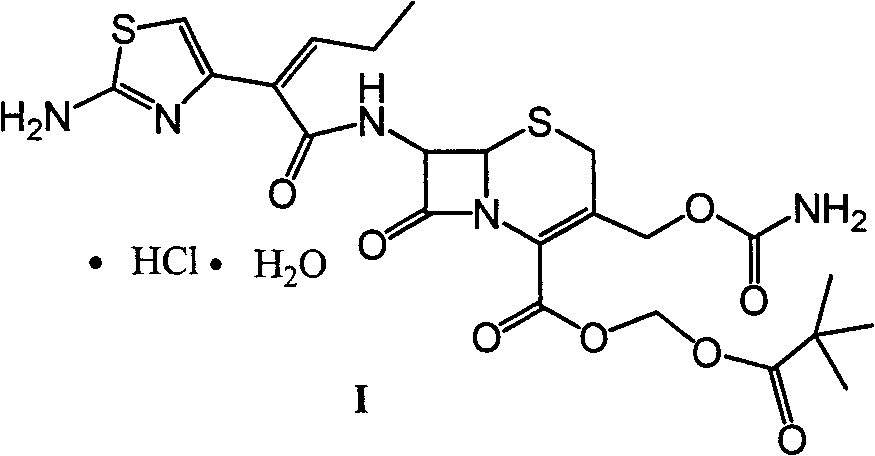
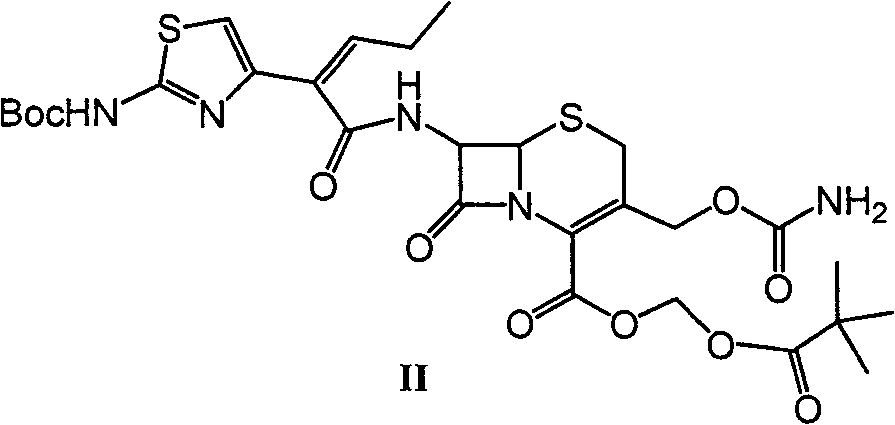
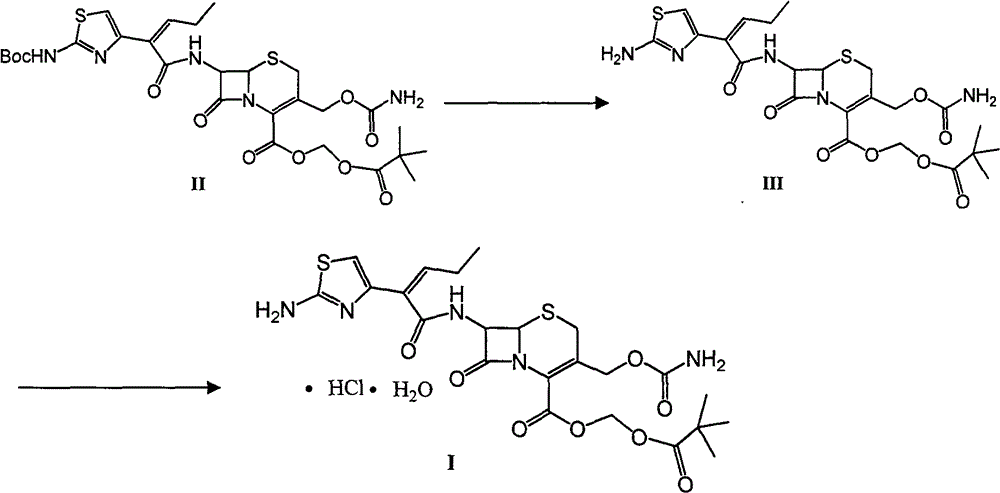
A kind of preparation method of cefcapene pivoxil hydrochloride and its synthetic intermediate
A technology of cefcapene pivoxil hydrochloride and cefcapene pivoxil, which is applied in the preparation method of cefcapene pivoxil hydrochloride and its synthetic intermediates, can solve problems such as cracking, difficult removal of phenol, and impact on product quality, and achieve mild conditions and high product quality. The effect of high quality and simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] (1) Preparation of Cefcapene Proxetil Sulfonate
[0033]
[0034] in:
[0035] R is C 1~12 Alkyl, phenyl, or substituted phenyl, the substituents are selected from C 1~12 Alkyl, C 1~12 Alkoxyl, C 1~12 Unsaturated alkyl halogen, amino; preferably methyl, ethyl, propyl, phenyl, p-methylphenyl; more preferably methyl, ethyl.
[0036] Sulfonic acid derivative is preferably commercially available product, can be pure product, also can be solution form, and sulfonic acid and tert-butoxycarbonyl cefcapene axetil mol ratio are 1~10: 1, are preferably 2~5: 1; Reaction The solvent is selected from one or more of acetone, tetrahydrofuran, methanol, ethanol, n-propanol, isopropanol, ethyl acetate, dichloromethane, acetonitrile, and water. The sulfonic acid used is different, and the reaction solvent is also different. The solid cefcapene axetil sulfonate can be obtained; the weight ratio of the reaction solvent to tert-butoxycarbonyl cefcapene axetil is 5-50:1, preferably 8...
Embodiment 1
[0048] Embodiment 1: the preparation of cefcapene pivoxil mesylate (IVa)
[0049] 100.0 g (0.15 mol) of Boc cefcapene pivoxil (II), 1.5 L of ethyl acetate and 24.7 mL (0.38 mol) of methanesulfonic acid were successively added into a 3 L reaction flask, and stirred at 30° C. for 8 hours. Filter, wash with ethyl acetate, and dry in vacuo to obtain 90.1 g of off-white solid with a molar yield of 90.5%, HPLC purity of 99.2%, and mp 230-232°C.
Embodiment 2
[0050] Embodiment 2: the preparation of cefcapene pivoxil ethanesulfonate (IVb)
[0051]100.0 g (0.15 mol) of Boc cefcapene pivoxil (II), 1 L of methanol and 38.6 mL (0.30 mol) of ethanesulfonic acid were successively added to a 3 L reaction flask, and stirred at 20° C. for 16 hours. Add 1 L of petroleum ether and stir at 20°C for 60 min. Filtration, washing, and vacuum drying yielded 93.8 g of off-white solid with a molar yield of 92.3%, HPLC purity of 99.3%, and mp of 165-167°C. 1H NMR (500MHz, DMSO-d6): δ9.43(d, J=7.5Hz, 1H); 8.80(brs, 1H); 6.65(s, 2H); 6.49(s, 1H); 6.25(t, J =7.5Hz, 1H); 5.89(d, J=6.0Hz, 1H); 5.80-5.83(m, 2H); 5.24(d, J=4.5Hz, 1H); 4.83(d, J=13.0Hz, 1H ); 4.59(d, J=13.0Hz, 1H); 3.65(d, J=18.5Hz, 1H); 3.54(d, J=18.0Hz, 1H); 2.50-2.51(m, 2H); 2.21-2.26 (m, 2H); 1.16(s, 9H); 1.10(t, J=7.5Hz, 3H); 1.02(t, J=7.5Hz, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com