Pharmaceutic adjuvant with collaborative work and anti-tumor application thereof
A technology for excipients and pharmaceutical compounds, applied in the field of pharmaceutical preparations, can solve the problems of loss of drug adaptability, no reported particle anti-tumor effect, low synthesis efficiency, etc., and achieve the effects of reducing treatment costs, good drug adaptability, and reducing toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of folic acid-dextran conjugates
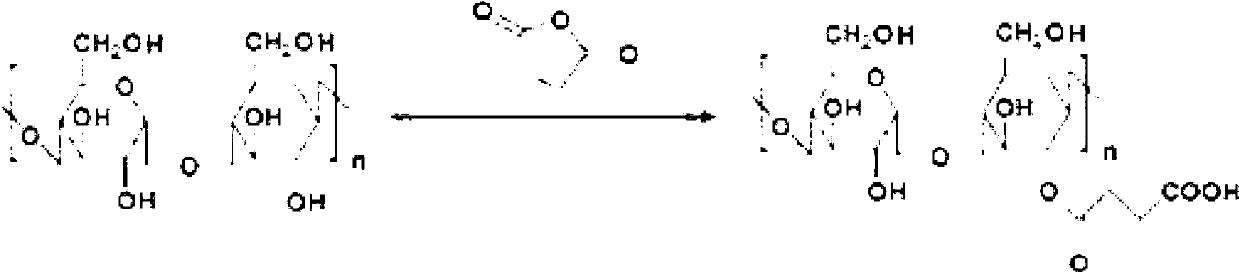
[0047] 1. Synthesis of Succinylated Dextran
[0048] The synthetic route is as follows:
[0049]
[0050] Take 10.0g dextran (average molecular weight 20KDa, Mw / Mn=1.3, available from Shanghai Sunshine Reagent Co., Ltd.) and dissolve it in anhydrous dimethylformamide (DMF), stir for 20 minutes to form a clear solution, and then add 1670 mg succinic anhydride and 200.0 mg 4-dimethylaminopyridine (DMAP), stir rapidly until all solids dissolve. Place at room temperature (25°C) to allow the entire reaction solution to react for 24 hours, then put the reaction solution into a Spectra / Por3 dialysis bag (molecular weight cut-off 3,500) and dialyze in water for 2 days. The solution in the dialysis bag was then freeze-dried to obtain white succinylated dextran. Nuclear magnetic resonance (NMR) results are as follows: 1H-NMR (d-DMSO): 2.8 (-CH 2 CH 2 -, succinylated dextran), 3.2-3.6 (dextran), 4.4-5.8 (dextran). ...
Embodiment 2
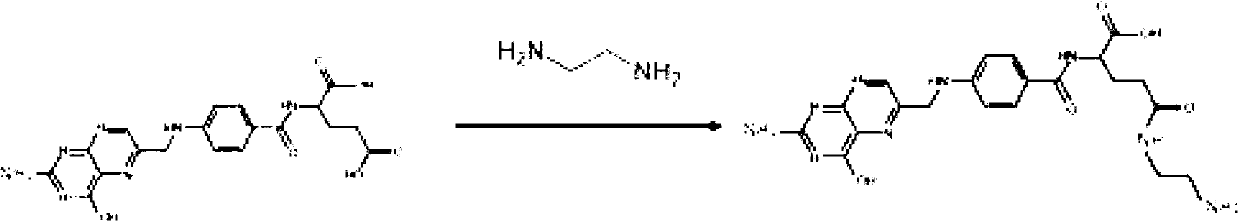
[0060] Example 2: Preparation and Stability Test of Freeze-dried Powder Injection of Paclitaxel Encapsulated by Folic Acid-Dextran Conjugate
[0061] Take 1000 mg of the folic acid-dextran conjugate prepared in Example 1 and dissolve it in 100 ml of 15 mM phosphate buffer (pH=7.4). After dissolving, place it on a magnetic stirrer and stir at 400 rpm. During the stirring process, dissolve 250 mg of paclitaxel in 5 mL of ethanol, add it dropwise to the stirred solution, and place it in a FS-1 high-speed homogenizer (available from Jintan Zhongzheng Instrument Manufacturing Co., Ltd.) , Homogenize 6 times at maximum power, 1 minute each time, stop for 2 minutes, then put the homogenate into Spectra / Por3 dialysis bag (molecular weight cut off 3,500), dialyze in 15mM phosphate buffer (pH=7.4) 12 hours to remove ethanol, then add 2500mg mannitol to adjust the osmotic pressure, and pass through a 0.2μm membrane filter to sterilize. After being detected by a particle size tester, the...
Embodiment 3
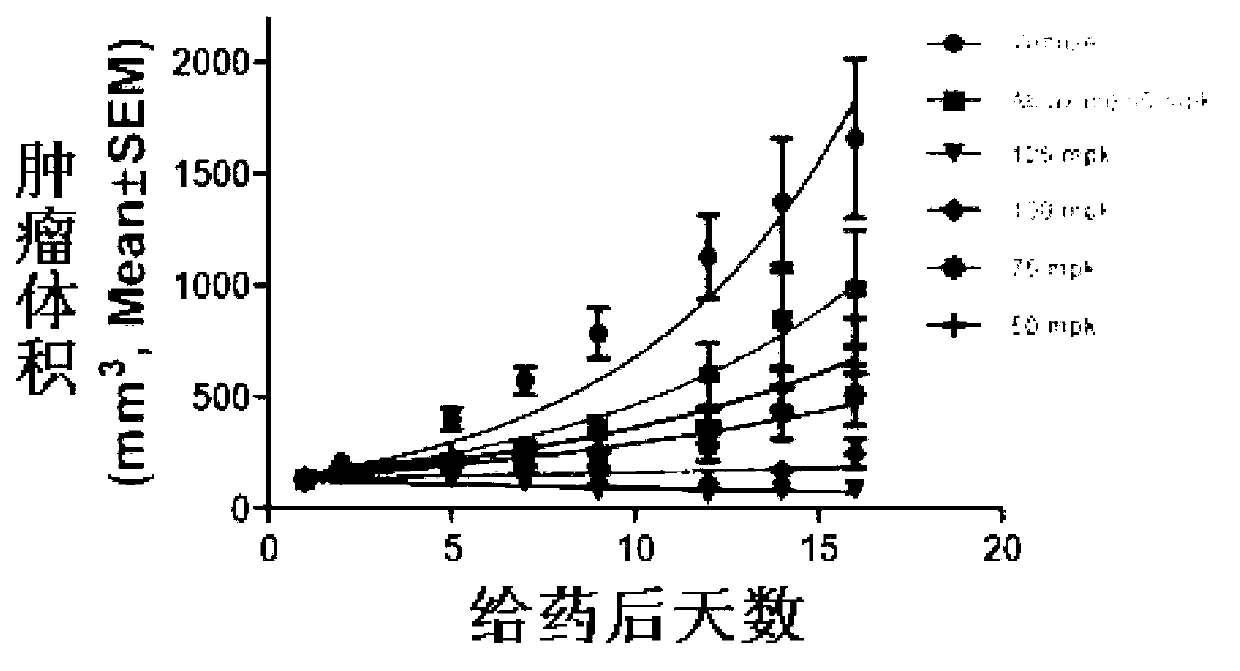
[0065] Example 3: Pharmacodynamic study of paclitaxel freeze-dried powder injection coated with folic acid-dextran conjugate
[0066] The tumor cytotoxicity test was carried out on the freeze-dried powder injection prepared in Example 2, and the tested cells were human non-lobular lung cancer cells. Freeze-dried powder injections of different concentrations were added to the IC50 was calculated after 72 hours of treatment in cell culture medium. The results showed that the cytotoxicity of paclitaxel encapsulated by various concentrations of folic acid-dextran conjugates was similar to that of paclitaxel at the same concentration without any treatment. The cytotoxicity of the glycoconjugate itself is very small, IC50>0.05mg / ml, it can be seen that the cytotoxicity in vitro mainly comes from paclitaxel, and the folic acid-dextran conjugate basically does not affect the cytotoxicity of paclitaxel.
[0067] The freeze-dried powder injection prepared by Example 2 using different...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com