Synthesis and application of envelope antigen universal for pyrethriods pesticide
A technology of pyrethroids and antigen-coated, applied to measuring devices, instruments, scientific instruments, etc., to meet the needs of research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
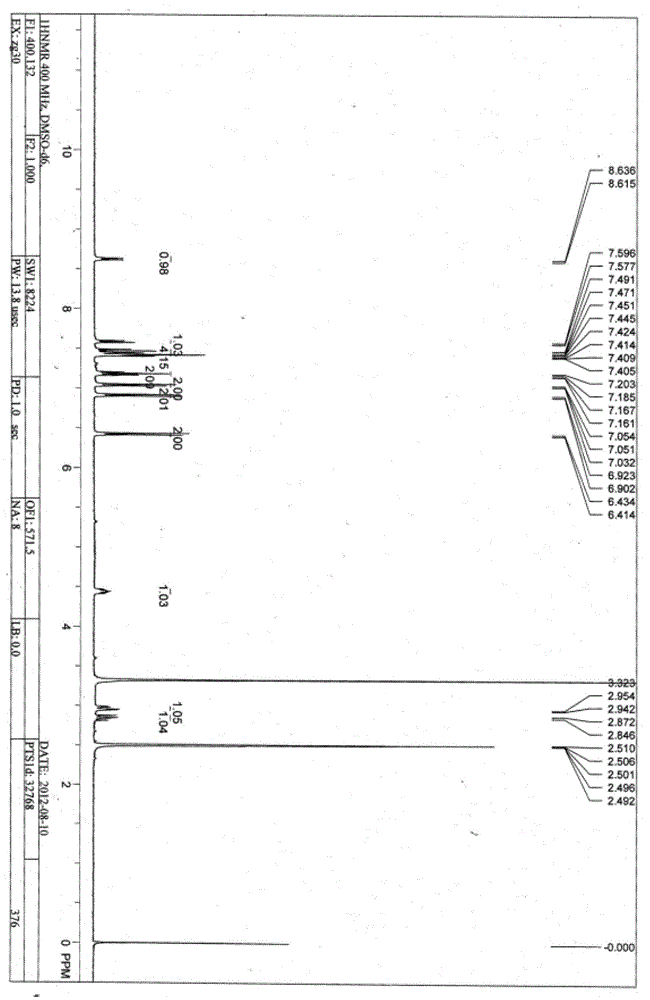
[0037] Example 1: Preparation of a universal coating antigen for pyrethroid pesticides
[0038] (1) Synthesis of haptens
[0039] ①Compound 3: Synthesis of N-(3-phenoxybenzoyl)-4-nitro-L-phenylalanine:
[0040] The reaction at N 2 Under protection, dissolve 500 mg of compound 1: 3-phenoxybenzoic acid in 10 mL of chloroform, place it in an ice bath after dissolving, then add 5 μL of DMF and 0.33 mL of thionyl chloride dropwise, stir for 5 min and then withdraw Remove from the ice bath, then heat in an oil bath at 65°C for 1 h, and remove chloroform and excess thionyl chloride by rotary evaporation to obtain a colorless and transparent liquid, which is dissolved by adding 1 mL of tetrahydrofuran to obtain liquid a; then add 480 mg of compound 2: Dissolve p-nitrophenylalanine and 380mg potassium carbonate in 10mL of water to form solution b; then slowly add solution a to solution b under vigorous stirring within 10 minutes, and after 1 hour of reaction, use 50mL ethyl acetate e...
Embodiment 2
[0058] 1. Preparation of coated plates
[0059] Use pH 9.6, 0.05M carbonate buffer solution to dilute the universal coating antigen of pyrethroid pesticides, doubling dilution 6 gradients, add 100 μL per well of the coating solution into a 96-well microtiter plate, overnight at 4 °C, Wash with washing solution (0.01M PBS containing 0.02% Tween20), each time pat dry on absorbent paper, and repeat four times. Then add 200 μL / well of blocking solution (pH 9.6, 0.05 M carbonate buffer solution containing 0.2% gelatin), place in a 37°C oven for 2 hours, take it out, wash it four times with the above washing solution, place in a 37°C oven for 15 minutes, and dry Store at 4°C after drying.
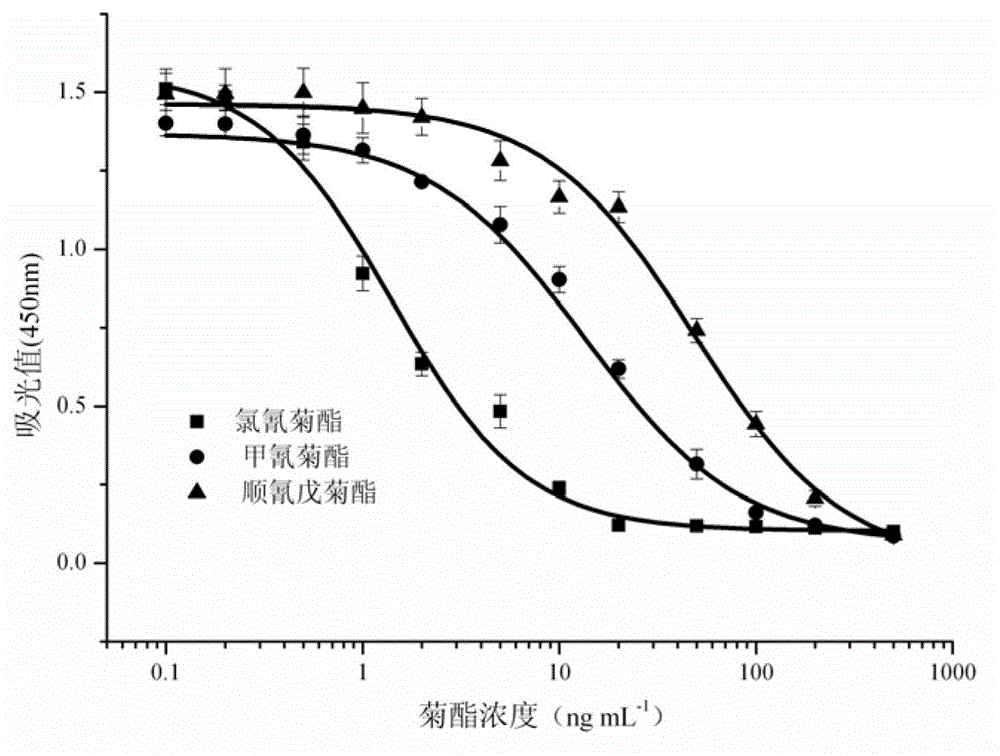
[0060] 2. Preparation of pyrethroid pesticide standard solution
[0061] Prepare a 1 mg / mL permethrin standard solution with n-hexane, place it in a brown glass bottle, and store it at -20°C. Before use, dilute the standard 10 times with n-hexane to 100 μg / mL. Measure 20 μL and place it in a ...
Embodiment 3
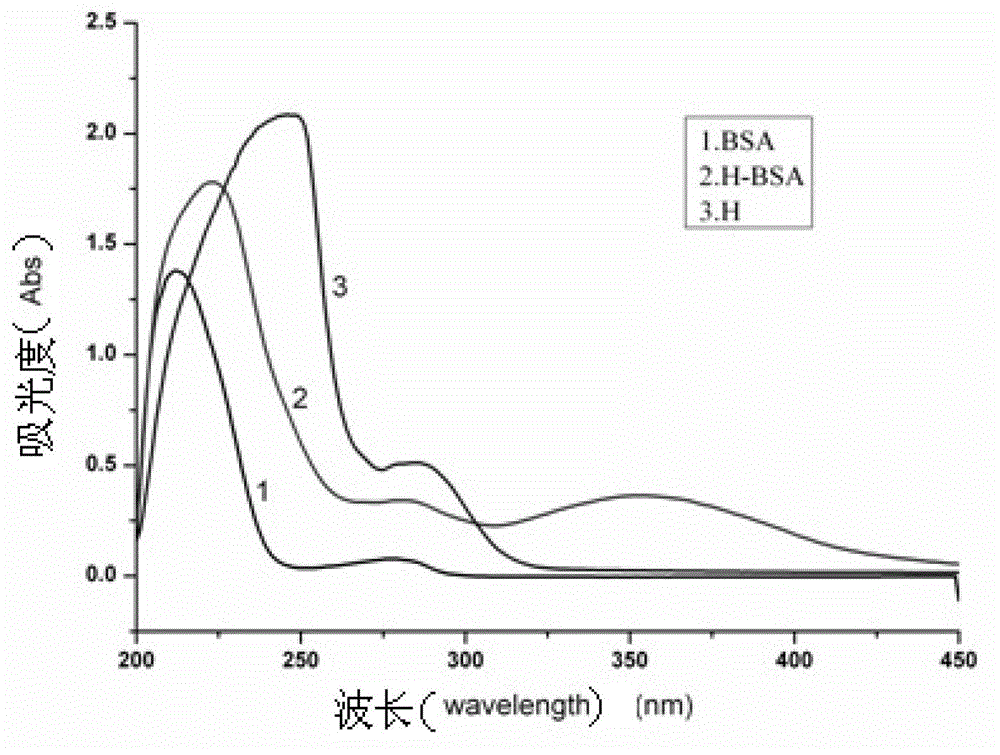
[0070]1 Coupling of gold nanoparticles and pyrethrin monoclonal antibody: The gold nanoparticles (particle size: 22-27nm) used in the present invention were synthesized by our laboratory. The specific method for coupling the group-selective antibody of pyrethroids to the surface of gold nanoparticles is: measure a certain amount of gold nanoparticle solution into a small test tube, and centrifuge (8000rpm, 10min), in order to remove citric acid in the gold nanoparticle solution Trisodium was resuspended with the same volume of ultrapure water, and then the pyrethroid group-selective antibody was slowly added dropwise into the gold nanoparticle suspension, shaken at room temperature for 2 hours, and then 1% BSA was added dropwise, so that the final concentration of BSA was 0.1%, shake at room temperature for 2 hours to block the unbound sites on the gold nanoparticles and avoid non-specific adsorption. Centrifuge (7000rpm, 8min) to remove excess antibody and BSA, resuspend in u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum absorption wavelength | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com