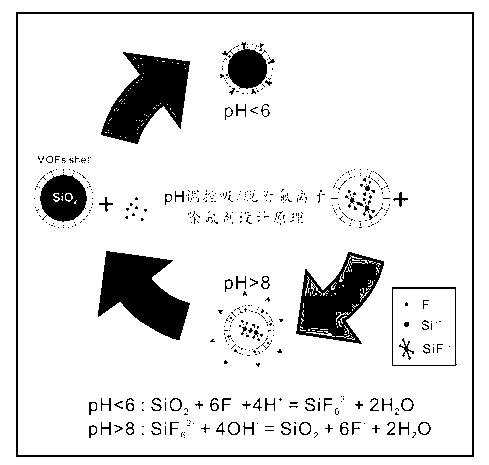
Fluorine removal agent with fluorine ion absorption/desorption function through pH value regulation and control and preparation method thereof
A technology for removing fluoride and fluoride ions, applied in chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve the problems of influence and secondary pollution, and achieve simple process, easy operation and low investment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
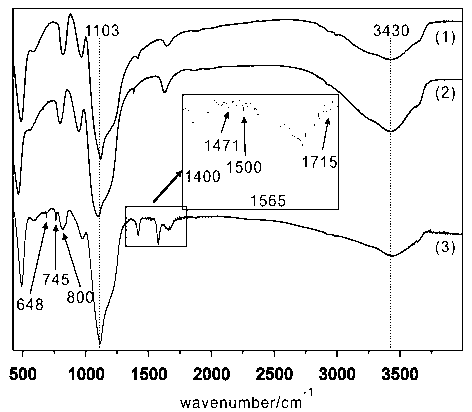
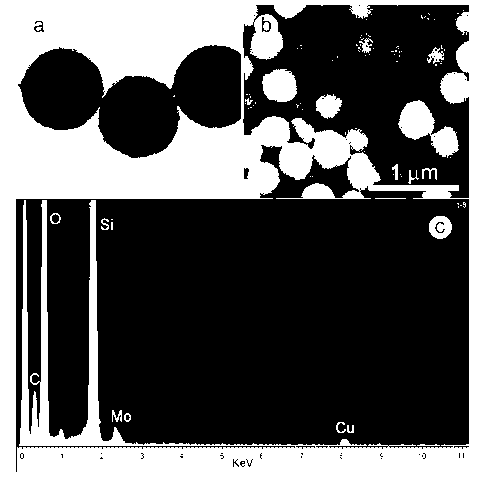
Image
Examples
Embodiment 1
[0038] (1) Surface activation of silica particles: Weigh 0.5 g of silica microspheres with a diameter of 250 nanometers, perform activation treatment under oxygen plasma for 30 minutes, and carry out carboxylation treatment in time for the activated silica microspheres;
[0039] (2) Carboxylation of the surface of silica particles: Dissolve 0.5 g of activated silica microspheres and 0.5 g of terephthalic acid in 20 ml of DMF solution, place the mixed solution in a 50 ml round bottom flask, and Ultrasound was used to dissolve it, and then 0.5 g of anhydrous sodium carbonate was added, and the mixture was stirred and reacted in an oil bath at 120°C for 2 hours. After cooling to room temperature, the product was obtained by centrifugation, washed several times with ethanol and water, dried and set aside;
[0040] (3) Growth of metal-organic framework shell on the surface of silica particles: at room temperature, weigh 0.5 g of carboxylated silica microspheres and disperse them in...
Embodiment 2
[0042] (1) Surface activation of silica particles: Weigh 0.5 g of silica microspheres with a diameter of 250 nm and place them in a prepared sodium hydroxide solution with a concentration of about 0.1 mol / L, and mix with 100 o Stir for 2 hours at C temperature, centrifuge, wash with deionized water 3 times, 60 o C under vacuum drying;
[0043] (2) Carboxylation of the surface of silica particles: Dissolve 0.5 g of activated silica microspheres and 0.5 g of terephthalic acid in 20 ml of DMF solution, place the mixed solution in a 50 ml round bottom flask, and Ultrasound was used to dissolve it, and then 0.5 g of anhydrous sodium carbonate was added, and the mixture was stirred and reacted in an oil bath at 120°C for 2 hours. After cooling to room temperature, the product was obtained by centrifugation, washed several times with ethanol and water, dried and set aside;
[0044] (3) Growth of metal-organic framework shell on the surface of silica particles: at room temperature, ...
Embodiment 3
[0046] (1) Surface activation of silica particles: Weigh 0.5 g of silica microspheres with a diameter of 250 nm and place them in a prepared sodium hydroxide solution with a concentration of about 0.1 mol / L, and mix with 100 o Stir for 2 hours at C temperature, centrifuge, wash with deionized water 3 times, 60 o C under vacuum drying;
[0047] (2) Carboxylation of the surface of silica particles: Weigh 0.2 g of activated silica microspheres and dissolve them in 10 ml of anhydrous N,N-dimethylformamide (DMF), then add 3-aminopropyl Triethoxysilane (APTMS) 2 ml, after the mixture is dissolved, at 120 o Stirring at C temperature for 6 hours, after the reaction, the obtained product was centrifuged and washed with DMF. The product obtained was dissolved in 10 milliliters of DMF solution, and another 0.9 gram of maleic anhydride was weighed and dissolved in 20 milliliters of ethanol solution, the two solutions were mixed and stirred at room temperature for 24 hours, and finally t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com