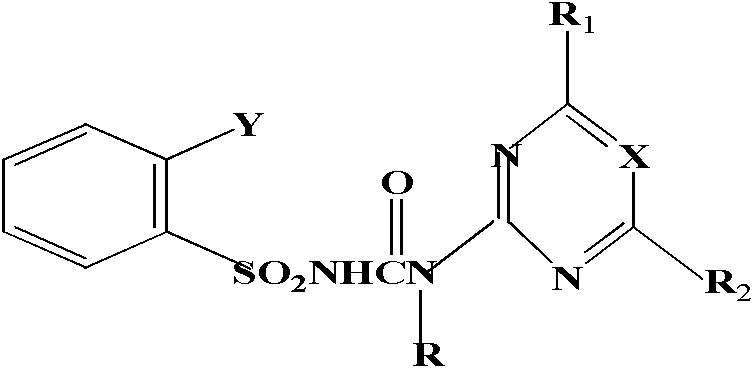
Bensulfuron-methyl universal hapten, artificial antigen, preparation method and application thereof
A bensulfuron-methyl, artificial antigen technology, applied in the preparation of sulfonic acid amides, biochemical equipment and methods, chemical instruments and methods, etc. Chemically stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation and Identification of Universal Hapten
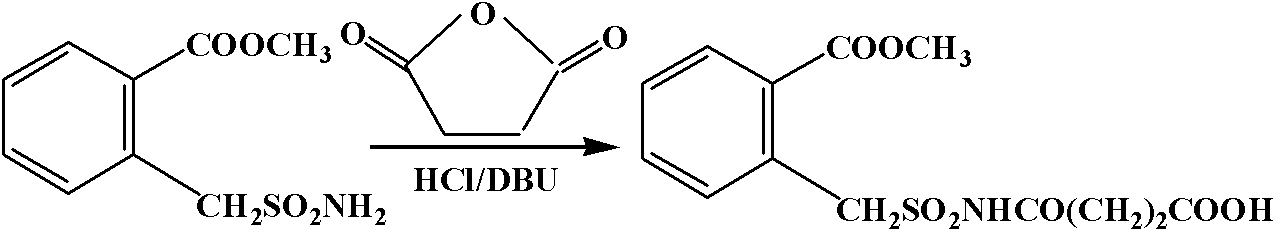
[0045] Dissolve 200mg of 2-methyl benzylsulfonamide 2-formate and 87mg of succinic anhydride in 5mL of acetonitrile, place in a water bath at 22°C, add 265.5mg of DBU dropwise within 10min, stir for 2h, then pour the reaction solution into 50mL of distilled water, 50mL Wash with ethyl acetate, remove the organic phase, adjust the pH of the aqueous phase to 2 with HCl, extract with ethyl acetate (2×50 mL), combine the organic phase with anhydrous MgSO 4 Dry, evaporate the solvent to dryness, and recrystallize from ethyl acetate to obtain Hapten, which is firstly detected by thin-layer chromatography (TLC), and then identified by NMR.
[0046] H NMR (CD 3 OD): δ7.91(d, 1H, J=7.5Hz Ar), 7.61-7.42(m, 3H, Ar), 4.78(s, 2H, CH 2 SO 2 ), 3.85(s, 3H, OCH 3 ), 2.48 (m, 2H, COCH 2 CH 2 COOH), 2.43(m, 2H, COCH 2 CH 2 COOH). See Figure 4 . Among them, some miscellaneous peaks appeared at 5.0-5.5 and 11-13 res...
Embodiment 2
[0047] Example 2 Synthesis and Identification of Artificial Antigen
[0048] Dissolve 16.45mg of Hapten in 1mL of tetrahydrofuran, add 13μL of tri-n-butylamine while stirring, after cooling to 0°C, add 7μL of isobutyl chloroformate, and stir for 60min under ice bath to obtain liquid A. Weigh 33.5 mg BSA (or 21.5 mg OVA) and dissolve it in borate buffer solution (0.2 mol / L, Ph=9.0) to form B solution. Slowly add solution A to solution B dropwise at 4°C, and stir overnight with magnetic force. The next day, the reaction solution was dialyzed in PBS buffer (pH=7.4) at 4°C for 3 days, the solution was changed 3 times / d, the dialysate was centrifuged at 10,000 r / min for 5 minutes, the supernatant was freeze-dried, and stored at -20°C.
[0049] The ultraviolet scanner scans the dialyzed sample at a wavelength of 190-400nm to identify the coupling situation. According to the characteristic absorption peak wavelength and absorbance value of each substance, the coupling ratio is calc...
Embodiment 3
[0052] Example 3 Preparation of Multiple Antiserum
[0053] The tail blood of Balb / c mice was observed for one week and kept as a negative control. The normal saline solution of the immunogen was mixed with Freund's complete adjuvant in equal amounts, and after the emulsification was complete, the mice were subcutaneously injected into the abdomen at multiple points, and the immune dose was 100 μg / 0.2ml. Two weeks later, incomplete Freund's adjuvant was used as emulsifier, and the mice were boosted with the same dose and method. The immunization process was once every two weeks, and immunized 4 times. 10 days after the third immunization, the mouse tail blood was collected, and the titer was measured by indirect non-competitive ELISA.
[0054] The inhibition rate of polyclonal antibodies against common haptens was determined by indirect competition ELISA (CI-ELISA). According to the optimal working concentration of antigen and antibody and optimized conditions, the general ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com