All-conjugate side-chain polymer and application thereof in polymer solar devices
A technology of conjugating side chains and polymers is applied in the field of organic polymer semiconductor materials, which can solve the problems of low carrier mobility and low matching degree of active layers, and achieve the effect of good performance and high efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of 1,3-dibromo-5-(4-octylphenyl)-5H-thiophene-[3,4-c]-pyrrole-3,6-dione, whose structural formula is:
[0041] .
[0042] Add 1.50 g of 4,6-dibromo-thiophene-[3,4-c]furan-1,3-dione, 0.44 g of 4-octylaniline and 0.72 g of 4-dimethylaminopyridine into a 100 mL flask, Add 35 ml of anhydrous 1,4-dioxane, stir and react for 20 hours under the protection of argon at 60°C, add 20 ml of glacial acetic acid, heat up to 80°C and continue stirring for 4 hours, pour into water after cooling, and use dichloro After extraction with methane, the organic layer was dried with anhydrous sodium sulfate and spin-dried, passed through the column with dichloromethane / petroleum ether = 1:5, and spin-dried to obtain 0.65 g of the target compound, with a yield of 61.4%. 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 7.28 (d, 2H), 7.23 (d, 2H), 2.63 (t, 2H), 1.62 (m, 2H), 1.36 – 1.29 (m, 10H), 0.88 (t, 3H), 13 C NMR (100 MHz, CDCl 3 ) δ (ppm): 155.9, 153.1, 149.4, 149.1, 148.7, 141.8...
Embodiment 2
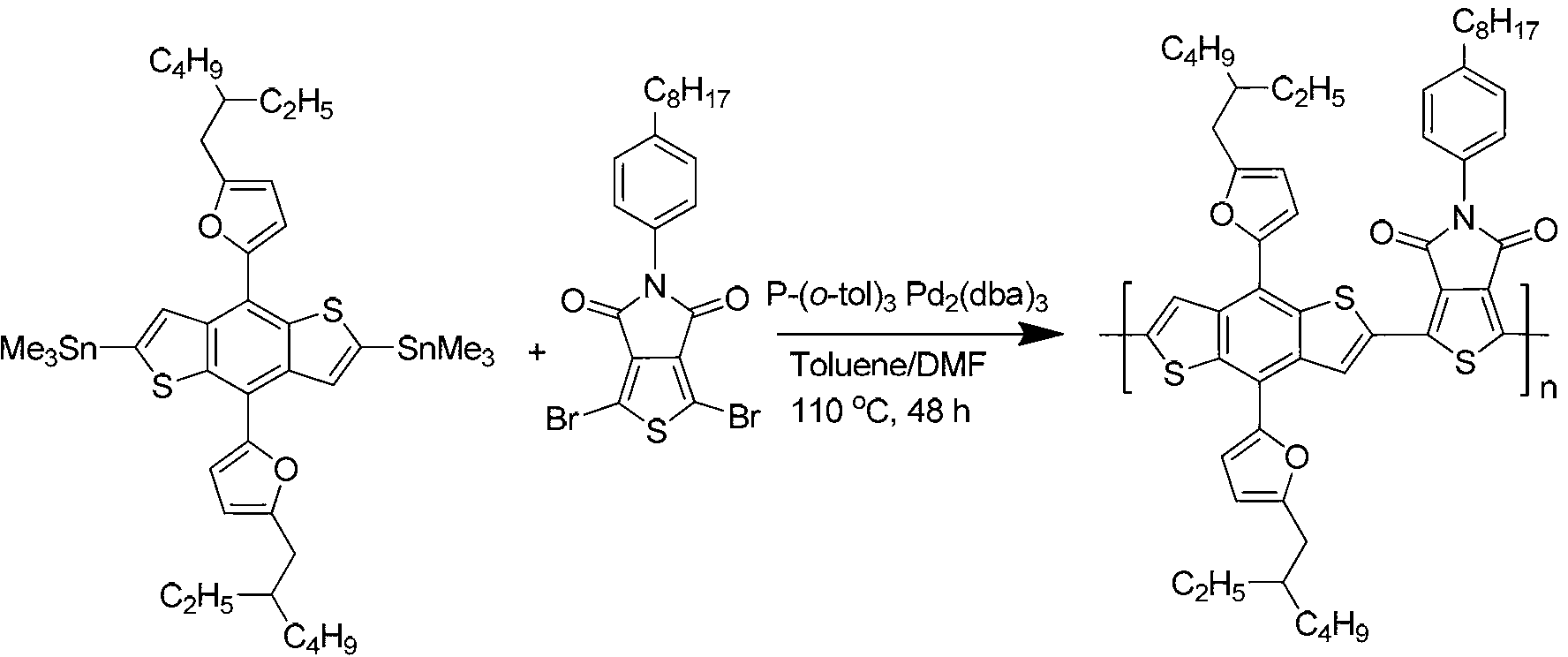
[0047] Polymer poly{[4,8-bis(5-(2-ethylhexyl)-thienyl)-benzo[1,2-b;4,5-b']dithieno-co-1, Synthesis of 3-dibromo-5-(4-octylphenyl)-5H-thiophene-[3,4-c]-pyrrole-3,6-dione (polymer PBDT-TT-P-TPD), Synthetic route such as figure 2 shown.
[0048] .
[0049] Take 0.24 g of 4,6-bis(trimethyltinyl)-4,8-bis(5-(2-ethylhexyl)-thienyl)-benzo[1,2-b;4,5- b']dithiophene, 0.12 g of 1,3-dibromo-5-(4-octylphenyl)-5H-thiophene-[3,4-c]-pyrrole-3,6-dione was added to 50 ml of reaction In the tube, add catalyst 0.01 g tris(dibenzylideneacetone) dipalladium, ligand 0.02 g tri-o-methylphenylphosphine, add 5 ml anhydrous toluene, 0.5 ml anhydrous N,N-dimethylformamide , stirred and reacted for 48 hours in an argon atmosphere at 110°C. Cool the polymer to room temperature and slowly pour it into 70 ml of methanol. The precipitated polymer is filtered and washed with methanol and n-hexane successively in a Soxhlet extractor. Finally, it is dissolved in chloroform and precipitated into methanol,...
Embodiment 3
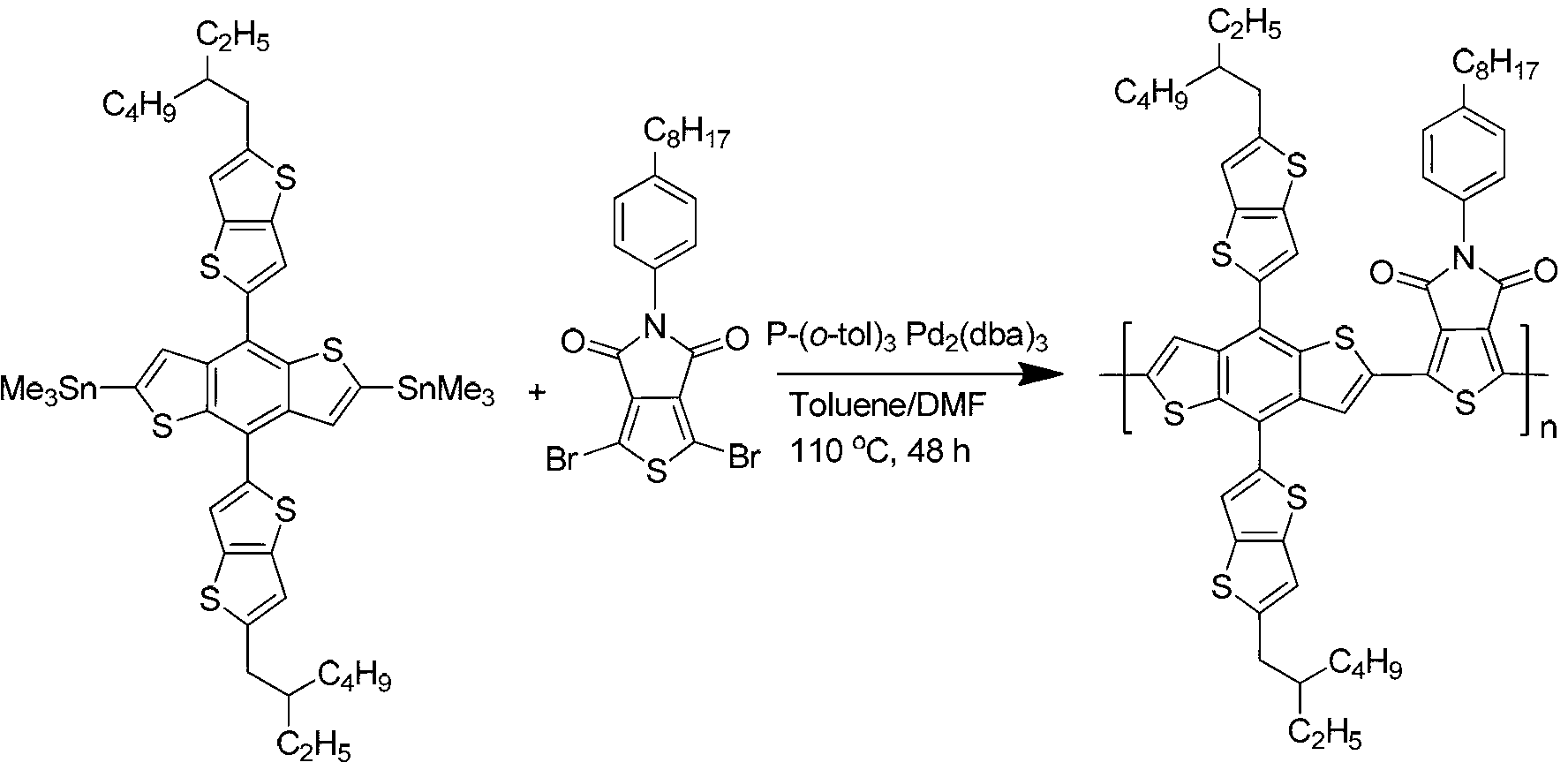
[0051] Polymer poly{[4,8-bis(5-(2-ethylhexyl)-thienyl)-benzo[1,2-b;4,5-b']dithieno-co-1,3 -Synthesis of dibromo-5-(4-octylphenyl)-5H-thiophene-[3,4-c]-pyrrole-3,6-dione (polymer PBDT-T-P-TPD), the synthetic route is as follows image 3 shown.
[0052] .
[0053] Take 0.21 g of 4,6-bis(trimethylstannyl)-4,8-bis(5-(2-ethylhexyl)-thienyl)-benzo[1,2-b;4,5-b ']dithiophene, 0.12 g of 1,3-dibromo-5-(4-octylphenyl)-5H-thiophene-[3,4-c]-pyrrole-3,6-dione into a 50 ml reaction tube Add catalyst 0.01 g tris(dibenzylideneacetone) dipalladium, ligand 0.02 g tri-o-methylphenylphosphine, add 5 ml anhydrous toluene, 0.5 ml anhydrous N,N-dimethylformamide, The reaction was stirred for 48 hours under an argon atmosphere at 110°C. Cool the polymer to room temperature and slowly pour it into 70 ml of methanol. The precipitated polymer is filtered and washed with methanol and n-hexane successively in a Soxhlet extractor. Finally, it is dissolved in chloroform and precipitated into methanol, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com