A kind of compound sulfamethoxazole injection and preparation method thereof
A technology of sulfamethoxazole and compound sulfonamide, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery, can solve the problems of high risk of organic solvents, complicated preparation methods, etc., and achieves improved antibacterial efficacy and absorption. Fast, stable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The preparation method of above-mentioned compound sulfamethoxazole injection comprises the following steps:
[0023] (1) Add sulfamethoxazole and trimethoprim to 20-30ml sodium hydroxide solution with a concentration of 0.2mol / l;
[0024] (2) Continue to add sodium hydroxide solution with a concentration of 0.4mol / l to adjust the pH value to 10-12 to completely dissolve sulfamethoxazole and trimethoprim;
[0025] (3) Continue to add tartaric acid or lactic acid to adjust the pH value to 3-4, and the solution in step (2) solidifies into a paste;
[0026] (4) Continue to add 0.2-1.0mol / l sodium hydroxide solution into the paste to completely dissolve the paste, and the pH value at this time is 6.5-7.5;
[0027] ⑸ adding antioxidants;
[0028] ⑹ adding chelating agent;
[0029] ⑺ Add water for injection, set the volume to 100 ml, and filter through a microporous membrane to obtain the finished product.
Embodiment 1
[0031] (1) Add 20g of sulfamethoxazole and 4g of trimethoprim into 20ml of sodium hydroxide solution with a concentration of 0.2mol / l, and stir;
[0032] (2) Continue to add 5ml of sodium hydroxide solution with a concentration of 0.4mol / l, adjust the pH value to 12, and completely dissolve sulfamethoxazole and trimethoprim;
[0033] (3) Continue to add 5g of tartaric acid or 5ml of lactic acid to solidify the solution in step (2) into a paste;
[0034] (4) Continue to add 10ml of 0.4mol / l sodium hydroxide solution to the paste to completely dissolve the paste;
[0035] (5) Add 0.2g of antioxidant;
[0036] ⑹Add 0.2g chelating agent;
[0037] (7) Add water for injection, set the volume to 100 ml, and filter through a 0.22um microporous membrane to obtain the finished product.
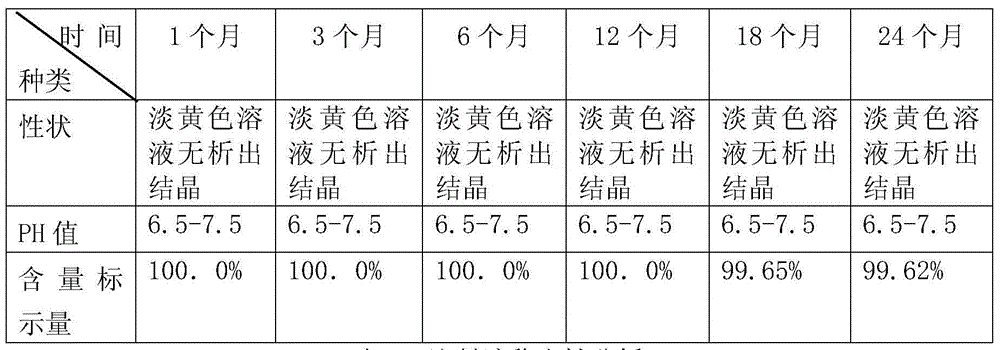
[0038] In this embodiment, the active ingredient sulfamethoxazole accounts for 20% of the total weight of the injection, and the pH of the injection is 6.8.
Embodiment 2
[0040] (1) Add 25g of sulfamethoxazole and 5g of trimethoprim into 20ml of sodium hydroxide solution with a concentration of 0.2mol / l, and stir;
[0041] (2) Continue to add 8ml of sodium hydroxide solution with a concentration of 0.5mol / l, adjust the pH value to 12, and completely dissolve sulfamethoxazole and trimethoprim;
[0042] (3) Continue to add 10g of tartaric acid or 10ml of lactic acid to solidify the solution in step (2) into a paste;
[0043] (4) Continue to add 10ml of 0.5mol / l sodium hydroxide solution to the paste to completely dissolve the paste;
[0044] (5) Add 0.2g of antioxidant;
[0045] ⑹Add 0.2g chelating agent;
[0046] (7) Add water for injection, set the volume to 100 ml, and filter through a 0.22um microporous membrane to obtain the finished product.
[0047] In this embodiment, the active ingredient sulfamethoxazole accounts for 25% of the total weight of the injection, and the pH of the injection is 7.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com