Method for producing cyclohexene through oxidative dehydrogenation of cyclohexane
A technology for oxidative dehydrogenation and cyclohexane, which is applied in the fields of chemical instruments and methods, organic chemistry, hydrocarbons, etc., can solve problems such as complex processing processes, and achieve the effects of simple operation, mild reaction conditions, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
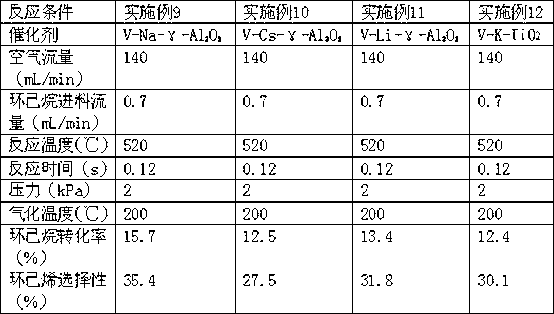
[0016] Example 1 Vanadium-potassium with γ-Al 2 O 3 Preparation of supported catalysts:
[0017] Accurately weigh 0.77g of ammonium metavanadate and 1.1g of oxalic acid and dissolve it in 20mL of distilled water, stir well at room temperature to dissolve all the ammonium metavanadate, and then add 20g of γ-Al 2 O 3 Put it into the solution, stir for 4 hours, and let it stand overnight. The obtained solution is subjected to rotary evaporation at 70 °C to remove excess water, and the catalyst obtained by rotary evaporation is dried in a drying oven at 120 °C for 12 hours. After drying, it was calcined in a muffle furnace at 650°C for 7h.
[0018] The catalyst prepared above was loaded with potassium, and 1.33 g of potassium nitrate was weighed and dissolved in 20 mL of distilled water, and fully stirred to dissolve it completely. The above catalyst was put into the solution and stirred for 4 hours, and left to stand overnight. The obtained solution was rotary-evaporated at 7...
Embodiment 2
[0020] Example 2 Vanadium-magnesium with γ-Al 2 O 3 Preparation of supported catalysts:
[0021] The preparation method is the same as above, wherein the amounts of ammonium metavanadate and magnesium nitrate used are 0.77g and 3.38g, respectively. Thus, V / Mg-γ-Al was prepared 2 O 3 The catalyst has a vanadium content of 1.5% by weight and a magnesium content of 1.5% by weight.
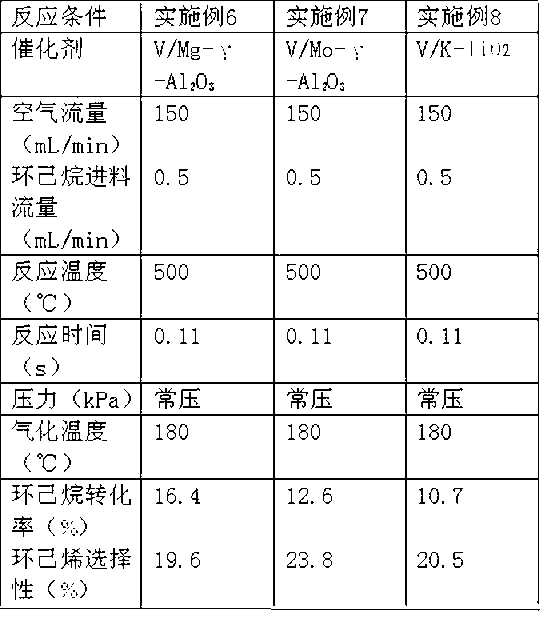
[0022] The above-mentioned catalyst is put into the fixed-bed reactor, the temperature of the control reactor is 500 ℃, and the normal pressure state; chamber to vaporize it. Thus, cyclohexane and air are mixed in the gasification chamber, and the reaction is carried out in the reactor, and the obtained product is analyzed by gas chromatography, and then the conversion rate of cyclohexane and the selectivity of cyclohexene are calculated to be 16.4% and 19.6%. .
Embodiment 3
[0023] Example 3 Vanadium-Molybdenum with γ-Al 2 O 3 Preparation of supported catalysts:
[0024] The preparation method is the same as above, wherein the amounts of ammonium metavanadate and ammonium molybdate used are 0.77g and 2.33g, respectively. Thus, V / Mo-γ-Al was prepared 2 O 3 The catalyst has a vanadium content of 1.6% by weight and a molybdenum content of 5.5% by weight.
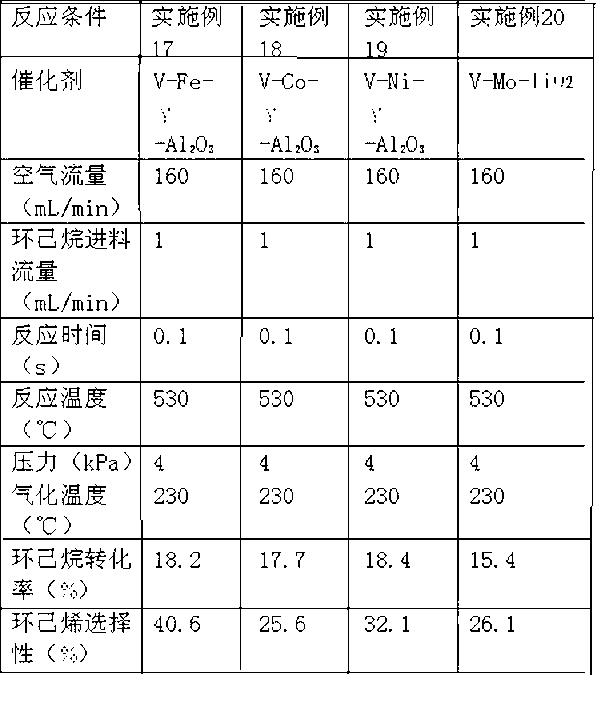
[0025] The evaluation method is the same as Example 1. The reaction conditions and reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com