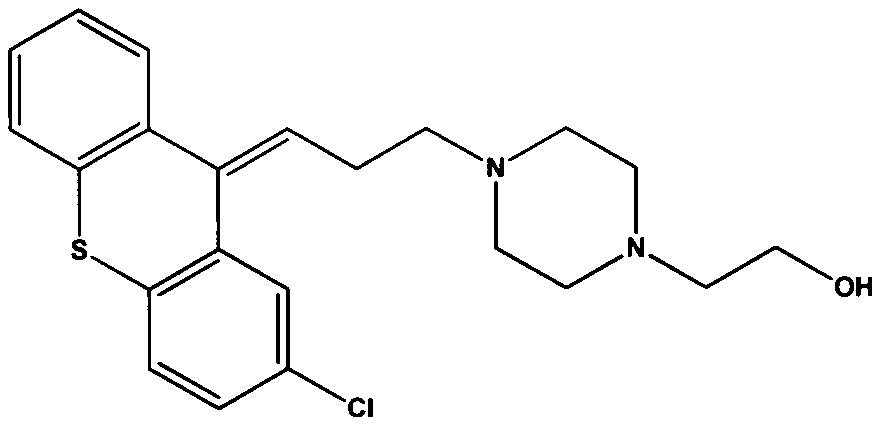
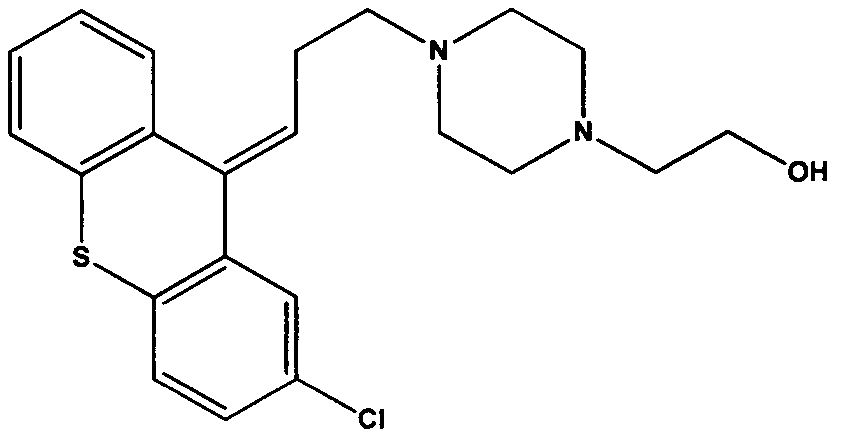
Separation and purification method of zuclopenthixol
A technology of zuclothixol and a purification method is applied in the field of improved separation of antipsychotic drug clothixol isomers, and purification of its α-isomer zuclothixol and its carboxylate esters to achieve high-yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
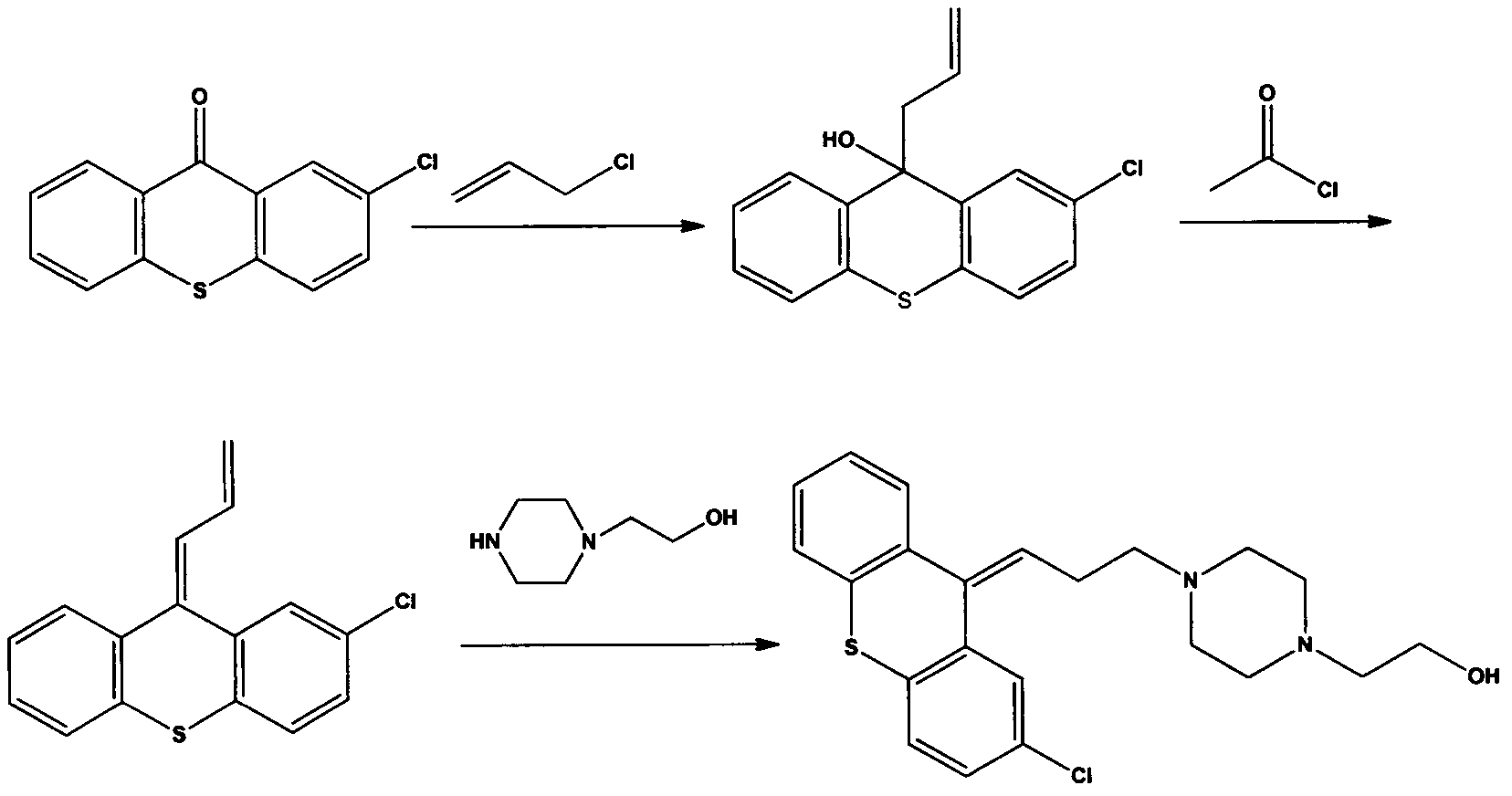
[0037] Embodiment 1: the preparation of chlorothixanthene base
[0038] 1) Preparation of 2-chloro-9-allyl-9-thioxanthol
[0039] Dissolve 100.00g (0.405mol) of 2-chloro-9-thioxanthone in 600mL of tetrahydrofuran, stir at 20°C-30°C, then add 26g of magnesium powder, 1g of iodine, and drop in 65g (0.855mol) of allyl chloride, React at 40°C-50°C for 2 hours, after cooling, add 1000ml of 20% sodium chloride aqueous solution dropwise to the reaction solution, stir for 10min, filter the insoluble matter, then extract twice with dichloromethane, 500ml each time, combine the organic phases, and water 500ml was washed, and the organic layer was separated, dried and filtered, and the filtrate was concentrated to remove the solvent to obtain 105.40g of 2-chloro-9-allyl-9-thioxanthol.
[0040] 2) Preparation of 2-chloro-9-(2-propenylene)thioxanthene
[0041] Dissolve 100.00g (0.346mol) of 2-chloro-9-allyl-9-thioxanthol in 100ml of toluene, heat the solution to 40°C, and dissolve 1.34g ...
Embodiment 2
[0044] Embodiment 2: Preparation of Zhuclothixol p-chlorobenzoate 2HCl
[0045] Dissolve 100.00g (0.250mol) of α / β-chlorothixanthene in 500ml of ethyl acetate, add dropwise 100ml of ethyl acetate dissolved in 52.48g (0.30mol) of p-chlorobenzoyl chloride at 40°C, drop After the addition was complete, the reaction was refluxed until the reaction was complete as monitored by TLC. 300ml of solvent was distilled off under reduced pressure, cooled to 4°C, and the precipitate was removed by filtration. The mother liquor was heated to 40°C, and 12.50g (0.125mol) of 37% concentrated hydrochloric acid aqueous solution was added dropwise. After reacting for about 1 hour, after cooling, a solid precipitated out, and 56.27g of cloclothixol p-chlorobenzoate·2HCl was obtained by filtration. Purity (HPLC as above) 97.11%, yield 36.82%.
Embodiment 3
[0046] Embodiment 3: the preparation of Zhuclothixol
[0047] Dissolve 45.25 g (0.074 mol) of chlorothixol p-chlorobenzoate·2HCl in 300 ml of 80% aqueous methanol, and then add 16.83 mol (0.30 mol) of potassium hydroxide. The mixture was heated to 50° C. and kept for 1 h. The solvent was distilled off under reduced pressure, extracted with toluene (200ml×2) and water, the organic phases were combined, concentrated under reduced pressure to remove the toluene; the obtained residue was recrystallized with cyclohexane to obtain 25.51 g of dry crystals. The purity was 99.7%, and the yield was 86.17%. 1 H NMR (CDCl 3 , 400MHz), δ: 7.10-7.46 (7H, m), 5.98 (1H, t), 3.41 (2H, t), 2.46-2.52 (14H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com