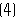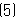Avermectin B2a/2b amine derivatives, derivative salts thereof, and preparation method and application of avermectin B2a/2b amine derivative salt
A technology of abamectin and derivatives, applied in the field of agrochemicals and preparation thereof, can solve problems such as unutilized B2a/2b
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
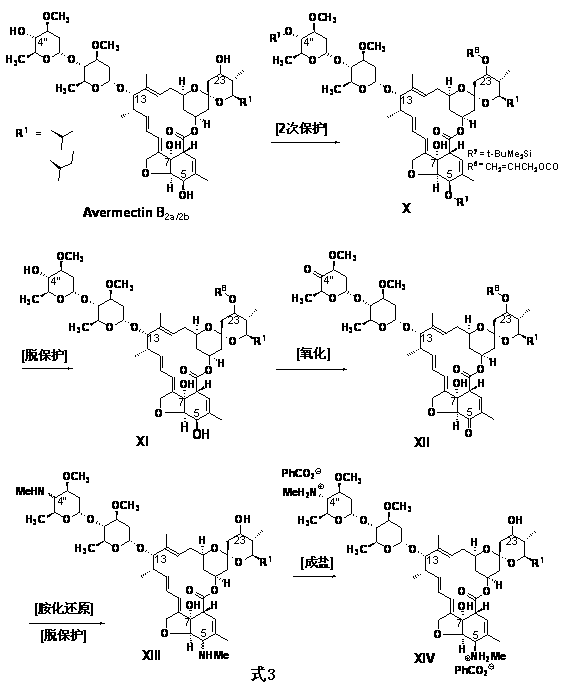
[0038] Example 1 4"-deoxy-4"-methylamino abamectin B 2a / 2b Preparation of benzoate
[0039] 10g abamectin B 2a / 2b ( Content B 2a 93%, B 2b 3% ) Dissolve in 100ml dichloromethane, lower the temperature to -30°C, add 3.0g allyl acetate and 3.0g tetramethylethylenediamine, and react for 2 hours. The temperature was raised to -20°C and then 5.0 g of tetramethylethylene diamine, 5.0 g of dimethyl sulfoxide and 5.0 g of phenyl phosphate dichloride were added, and the reaction was carried out for 3 hours. Add 5% phosphoric acid solution and 10% sodium hydroxide solution respectively, adjust the pH value to 2 and then to 7-8, separate the layers, dry the organic phase with sodium sulfate, and remove the solvent under reduced pressure. The residue was dissolved in toluene, 10g heptamethyldisilazane and 1.0g zinc chloride were added, and the temperature was kept at 75°C for 6 hours. Start to cool down, add 10g ethanol, 2.0g sodium borohydride when the temperature is cooled to 0°C, keep...
example 2
[0040] Example 2 23-deoxy-23-methylaminoabamectin B 2a / 2b Preparation of phosphate
[0041] 10g Abamectin B 2a / 2b (Content B 2a 93%, B 2b 3%) Dissolve in 100m1 of dichloromethane, lower the temperature to -30°C, add 3.0g tert-butyldimethylchlorosilane and 3.0g tetramethylethylenediamine, and react for 2 hours. The temperature was raised to -20°C and 8.0 g of tetramethylethylene diamine, 8.0 g of dimethyl sulfoxide and 8.0 g of phenyl phosphate dichloride were added, and reacted for 3 hours. Add phosphoric acid solution and sodium hydroxide solution, adjust the pH to 2 and then to 7-8, separate the layers, dry the organic phase with sodium sulfate, and remove the solvent under reduced pressure.
[0042] The residue was dissolved in toluene, 10g heptamethyldisilazane and 1.0g zinc chloride were added, and the temperature was kept at 75°C for 6 hours. Start to cool down, add 10g ethanol, cool to 0°C, add 2.0g sodium borohydride, 1 hour later, add 10g ethanol, 0.005g tetrakistriphen...
example 3
[0043] Example 3 4",23-deoxy-4",23-bis(N-methylamino)abamectin B 2a / 2b Preparation of benzoate
[0044] 10g abamectin B 2a / 2b ( Content B 2a 93%, B 2b 3% ) Dissolve in 100ml dichloromethane, lower the temperature to -30°C, add 1.5g tert-butyldimethylchlorosilane and 1.0g tetramethylethylenediamine, and react for 2 hours. The temperature was raised to -20°C and 8.0 g of tetramethylethylene diamine, 8.0 g of dimethyl sulfoxide and 8.0 g of phenyl phosphate dichloride were added, and reacted for 3 hours. Add phosphoric acid solution and sodium hydroxide solution, first adjust the pH to 2 and then to 7-8, separate the layers, dry with sodium sulfate, and remove the solvent from the organic phase under reduced pressure.
[0045] The residue was dissolved in toluene, 15g heptamethyldisilazane and 1.5g zinc chloride were added, and the temperature was kept at 75°C for 6 hours. Start to cool down, add 10g ethanol, add 4.0g sodium borohydride and keep it for 1 hour when the temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com