Integrin targeting drug loaded albumin nanoparticle formulation and its preparation method
A technology of albumin nanoparticles and albumin, which is applied in the field of gemcitabine albumin nanoparticles and its preparation, and integrin-targeted drug-loaded albumin nanoparticles preparations, which can solve the problem of inability to be used as a directional carrier and insufficient expression of pancreatic cancer cells. High, no specificity and other issues, to achieve good tumor targeting, improve treatment effect, reduce the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]Example 1 Preparation of RGD-Coupled Gemcitabine Albumin Nanoparticles
[0031] Prepare 10ml of 3.0% BSA solution and adjust the pH to 8.5 with 1M NaOH. Add 40ml of absolute ethanol dropwise at a rate of 1ml / min under high-speed stirring until a blue gel-like solution is formed, and continue stirring for 60min. Add cross-linking agent glutaraldehyde (albumin amino molar ratio is 100%) under constant stirring, and continue stirring at a constant speed for 16-20 hours. Ethanol was removed by rotary evaporation at low temperature (40° C.), passed through a 1 μm filter membrane, and the remaining colloidal solution was centrifuged at 12,000 rpm for 10 minutes. The supernatant was removed, and the precipitated part was redissolved with distilled water to obtain a suspension of albumin nanoparticles. Take 5mg of RGD polypeptide, take a small amount from it and place it in a sterilized 1.5ml centrifuge tube, use 100μl double distilled water to try to dissolve the polypeptide,...
Embodiment 2
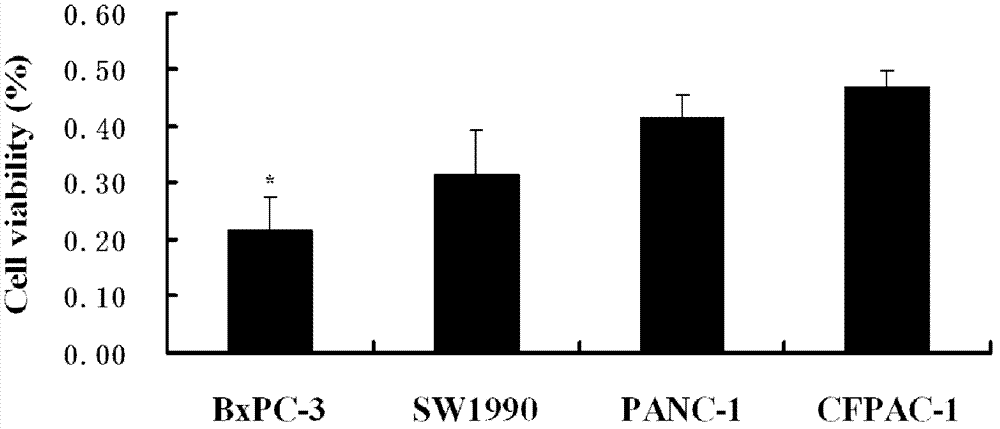
[0033] Example 2 RGD-Coupled Gemcitabine Albumin Nanoparticles Pairs Expressing Different Integrin α v beta 3 Pancreatic Cancer Cell Line Inhibition Experiment
[0034] The positive expression rates of integrin αvβ3 receptors on the surface of PANC-1, BxPC-3, SW1990, and CFPAC-1 cell lines in logarithmic growth phase were 13.58%, 86.94%, 71.50%, and 12.51%, respectively. The adherent cultured pancreatic cancer cell lines in the logarithmic growth phase were digested with 0.05% trypsin, and made into single-cell suspension with RPMI 1640 culture medium containing 10% calf serum. 3 The cells were inoculated into the wells of 96-well culture plates, with a volume of 200 μl per well. Move the culture plate to CO 2 In an incubator at 37°C, 5% CO 2 And greater than 95% humidity after cultivating for 24 hours, the cells adhered to the wall, and the cells were nearly fused. The culture solution was sucked out, and the RPMI 1640 culture solution containing 0.2% calf serum was left ...
Embodiment 3
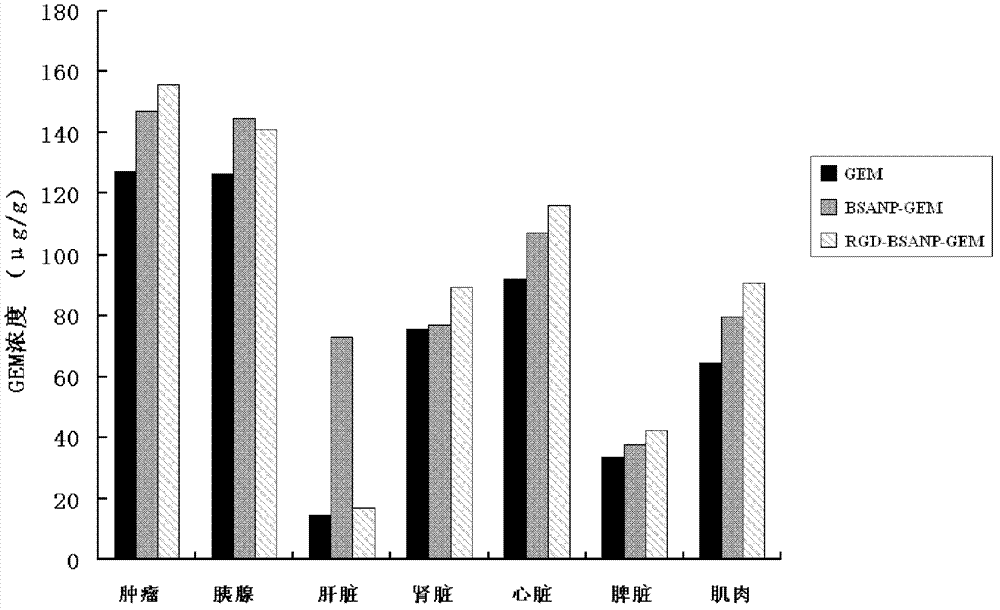
[0035] Example 3 Tissue Drug Concentration Distribution of RGD-Coupled Gemcitabine Albumin Nanoparticles in Nude Mouse Implanted Tumor Model
[0036] Fifteen tumor-bearing nude mice were selected and randomly divided into 3 groups: GEM original drug group, BSANP-GEM group, and RGD-BSANP-GEM group. They were injected into the tail vein, and the dosage was calculated as GEM, which was GEM 90 mg / kg. 30 minutes after the administration, the nude mice were killed, and two pieces of tumor tissue, liver, kidney, spleen, pancreas, heart, and muscle tissue of 0.5 cm*0.5 cm were collected respectively. After the tissue was homogenized, the concentration of gemcitabine in the tissue was detected by HPLC. Chromatographic conditions:: chromatographic column: Phenomenex C18 (250mm×4.6mm, 4μm); column temperature: 45°C; mobile phase: acetonitrile-0.1% trifluoroacetic acid solution (3:97, v / v); flow rate: 1.0mL / min; UV detection wavelength: 268nm; injection volume: 100μL. The drug concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com