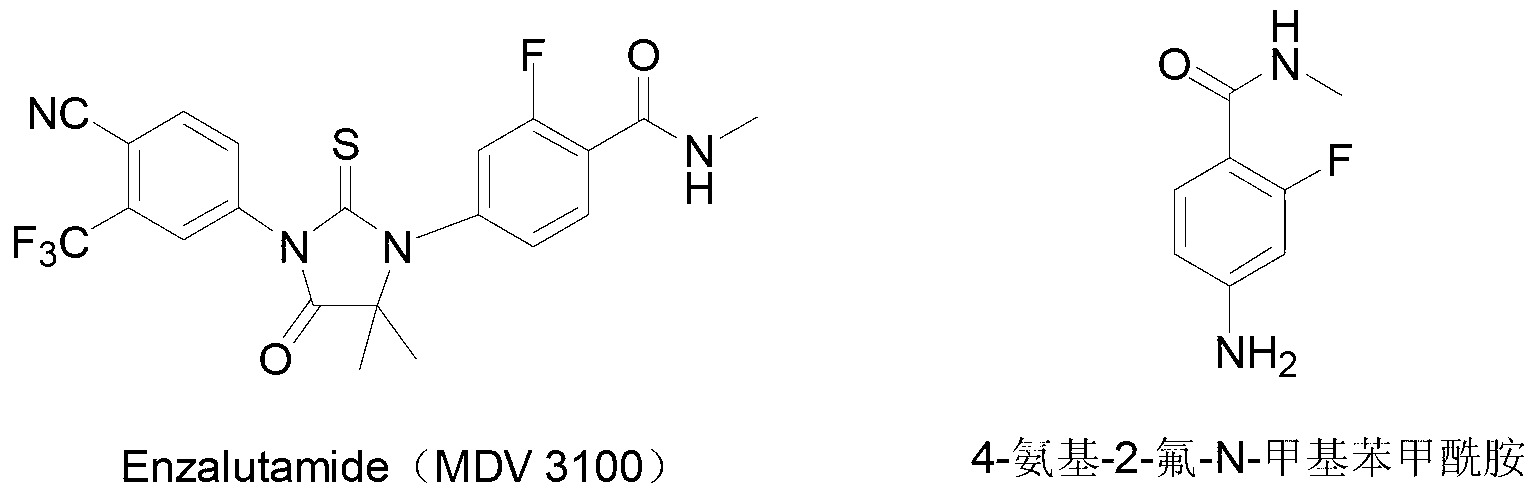
Preparation method of 4-amino-2-fluoro-methyl benzamide
A technology of methyl benzamide and nitrotoluene, applied in the preparation of carboxylic acid amide, preparation of organic compounds, chemical instruments and methods, etc., can solve environmental pollution and other problems, achieve good purity, less waste water, and mild reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
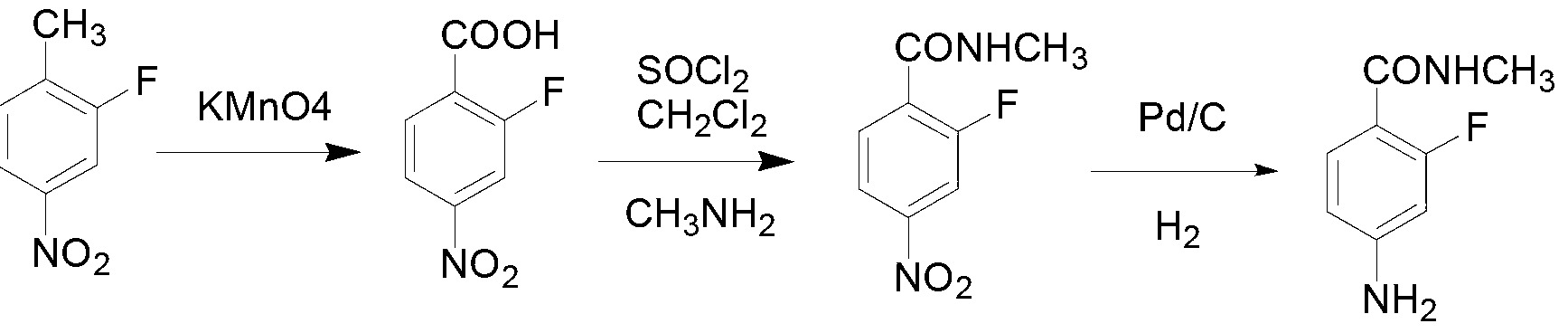
[0026] Preparation of 2-fluoro-4-nitrobenzoic acid
[0027] Add 2-fluoro-4-nitrotoluene (31.1g, 0.2mol), water (500ml), sodium hydroxide (10.0g, 0.25mol) and tetrabutylammonium bromide (3.2g, 0.01 mol), stir evenly and heat to 95°C, add potassium permanganate (79.0g, 0.5mol) in batches under stirring, react at 85°C for 8 hours, follow the reaction by TLC, filter while hot to remove di Manganese oxide, the filtrate was adjusted to pH 2 by adding concentrated hydrochloric acid, a white precipitate was precipitated, left to stand, filtered, washed with water, and dried to obtain off-white solid 2-fluoro-4-nitrobenzoic acid (27.3g), the yield was 73.7% (with 2 -Fluoro-4-nitrotoluene), with a content of more than 98%, 1 H NMR: (400Hz, DMSO-d), δ (ppm), 8.23 (d, J=10.5Hz, 1H), 8.06-8.18 (m, 2H);
[0028] Other conditions remain unchanged, without adding sodium hydroxide and tetrabutylammonium bromide, when potassium permanganate is used to directly oxidize 2-fluoro-4-nitrobenzoi...
Embodiment 2
[0035] Preparation of 2-fluoro-4-nitrobenzoic acid
[0036] Add 2-fluoro-4-nitrotoluene (31.1g, 0.2mol), water (1000ml), sodium hydroxide (8.0g, 0.2mol) and triethylbenzyl ammonium chloride (4.56 g, 0.02mol), stir evenly and heat to 75°C, add potassium permanganate (94.8g, 0.6mol) in batches under stirring, react at 80°C for 18 hours, follow the reaction by TLC, and complete the reaction while hot Remove manganese dioxide by filtration, add concentrated hydrochloric acid to the filtrate to adjust the pH to 4, a white precipitate precipitates out, let stand, filter, wash with water, and dry to obtain off-white solid 2-fluoro-4-nitrobenzoic acid (27.9g), yield 75.3% (calculated as 2-fluoro-4-nitrotoluene), the content is more than 98%, 1 H NMR: (400Hz, DMSO-d), δ (ppm), 8.23 (d, J=10.5Hz, 1H), 8.06-8.18 (m, 2H);
[0037] Preparation of 2-fluoro-4-nitro-N-methylbenzamide
[0038] Add 2-fluoro-4-nitrobenzoic acid (27.9g, 0.15mol), chloroform (450ml), triethylamine (1.0ml) int...
Embodiment 3
[0042] Preparation of 2-fluoro-4-nitrobenzoic acid
[0043] Add 2-fluoro-4-nitrotoluene (31.1g, 0.2mol), water (500ml), sodium hydroxide (20.0g, 0.50mol) and tetrabutylammonium chloride (6.22g, 0.022 mol), stir evenly and heat to 80°C, add potassium permanganate (64.8g, 0.41mol) in batches under stirring, react at 95°C for 16 hours, follow the reaction by TLC, filter while hot to remove di Manganese oxide, the filtrate was adjusted to pH 2 with concentrated hydrochloric acid, a white precipitate precipitated, left to stand, filtered, washed with water, and dried to obtain off-white solid 2-fluoro-4-nitrobenzoic acid (27.5g), yield 74.2 (with 2- Fluoro-4-nitrotoluene), the content is more than 98%.
[0044] Preparation of 2-fluoro-4-nitro-N-methylbenzamide
[0045] Add 2-fluoro-4-nitrobenzoic acid (27.5g, 0.148mol), 1,2-dichloroethane (275ml), N,N-dimethylformamide (1.38ml) into a 1L reaction flask, Add thionyl chloride (26.3g, 0.22mol) dropwise, stir at room temperature for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com