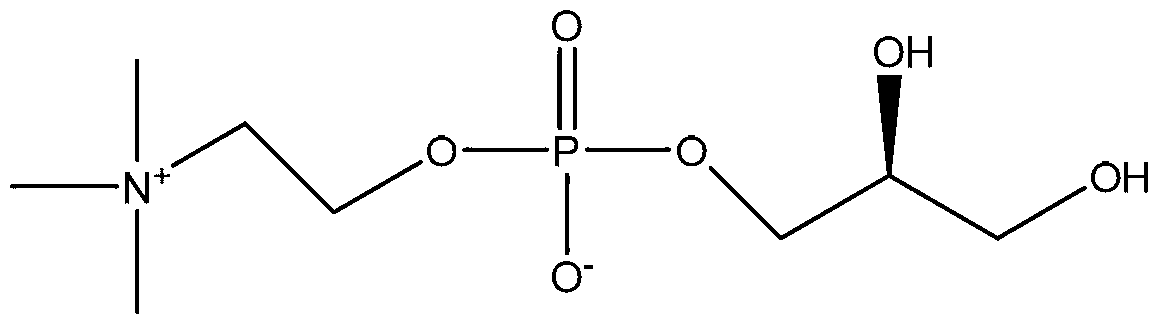
Preparation method of L-alpha-glycerophosphoryl choline
A technology of glycerophosphocholine and chlorinated glycerophosphorylcholine benzyl ether, which is applied in the field of preparation of chiral drugs, can solve the problems of low yield, uneven impurity composition, large waste water discharge, etc., and achieve high yield , the effect of good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
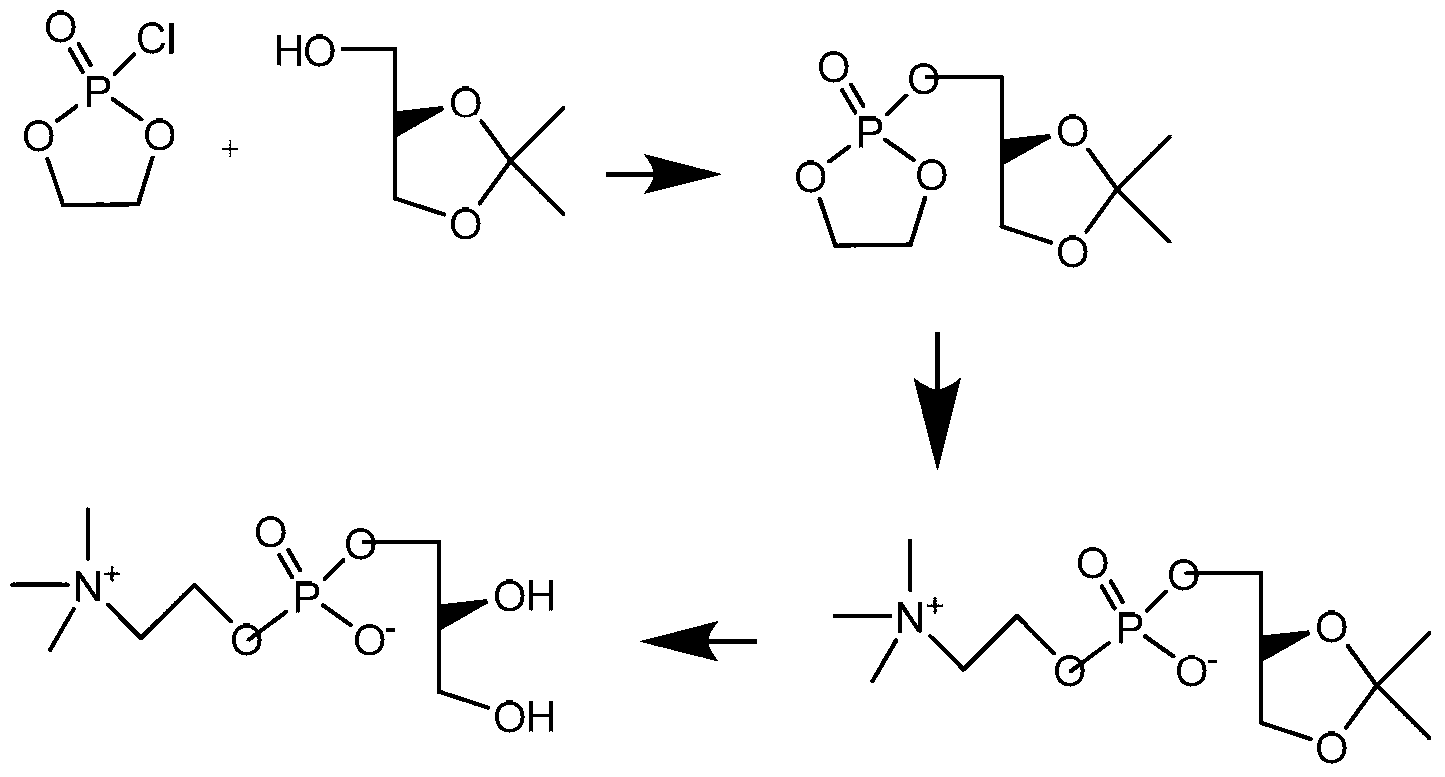
Embodiment 1
[0038] Preparation of (S)-Benzyl Glycidyl Ether
[0039]
[0040] Take 324.4 grams (3mol) of benzyl alcohol and 60 grams (1.5mol) of sodium hydroxide, put them into a 500ml reaction bottle, stir and heat to 60-70°C, then add 92.5 grams of (R)-epichlorohydrin (1mol) dropwise, Control the reaction temperature around 65°C. After about 2 hours of dripping, keep the reaction at 60-70°C for 12 hours, and gas chromatography detects that the raw material (R)-epichlorohydrin has completely reacted. Cool to room temperature, adjust the pH value to 6-7 with dilute hydrochloric acid, and filter. The filtrate was washed with water (300mlX2). After separating the water layer, dry it overnight with anhydrous sodium sulfate, filter, collect the filtrate to vacuum distill excess benzyl alcohol, and then high vacuum distill the product (S)-benzyl glycidyl ether 130.5 g, the yield of this step is 80%.
Embodiment 2
[0042] Preparation of L-α-Glycerophosphocholine Benzyl Ether Chloride
[0043]
[0044] 146 g (0.5 mol) of phosphorylcholine chloride and 500 ml of absolute ethanol were heated under reflux until completely dissolved. Then 82 g (0.5 mol) of (S)-benzyl glycidyl ether was added dropwise. About 1.5 to 2 hours to drop. The reflux reaction was continued for 18 hours, and the reaction of (S)-benzyl glycidyl ether was detected by TLC to be complete. Atmospheric distillation recovers ethanol solvent. A colorless oil was obtained, which was directly put into the next reaction without further purification.
Embodiment 3
[0046] Preparation of L-α-Glycerophosphocholine Chloride
[0047]
[0048]The product from the previous step was transferred to a high-pressure reactor, 800 ml of ethanol, 20 g of 10% Pd / C catalyst were added, and high-pressure hydrogen was fed to 10 atmospheres. The reaction was closed and stirred for 24 hours until no hydrogen was absorbed. Open the hydrogen valve to release the hydrogen slowly. The reaction solution was filtered, and the ethanol solvent was recovered by normal pressure distillation to dryness, then 500 ml of ethanol was added, and the normal pressure distillation was again to dryness. A colorless oil was obtained. Then 400 ml of absolute ethanol and 100 ml of isopropanol were added and stirred at 0-3° C. for 20 hours to slowly crystallize. Filter and wash the filter cake with a small amount of ethanol. After vacuum drying, 113.6 g of the product was obtained, and the two-step yield was 78%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com