Compound tilmicosin injection, applications, application method and preparation method
A technology of tilmicosin and injection, applied in the field of compound tilmicosin injection, can solve problems such as inability to treat porcine respiratory disease syndrome in a targeted manner, achieve normal blood volume, maintain normal blood pressure, relieve The effect of body heat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] S101: By weight percentage, 12 parts of alcohol, 23 parts of dimethylethanolamine, 0.6 part of diaobaikuai, and 6 parts of ethanolamine.
[0036] S102: Take 35 parts of propylene glycol, add all the above components into the propylene glycol to form a mixed solution, and stir.
[0037] S103: Take 31 parts of tilmicosin, 6 parts of doxycycline, 6 parts of flunixin meglumine, and 0.07 part of dexamethasone, add them to the above mixture, and stir to make a compound tilmicosin injection.
[0038] S104: The compound tilmicosin injection is used to treat porcine respiratory syndrome.
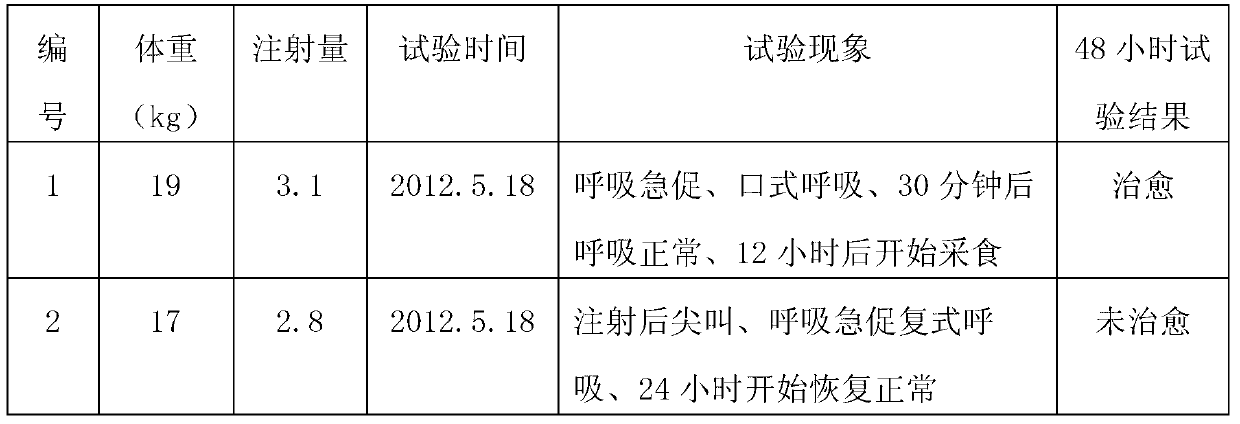
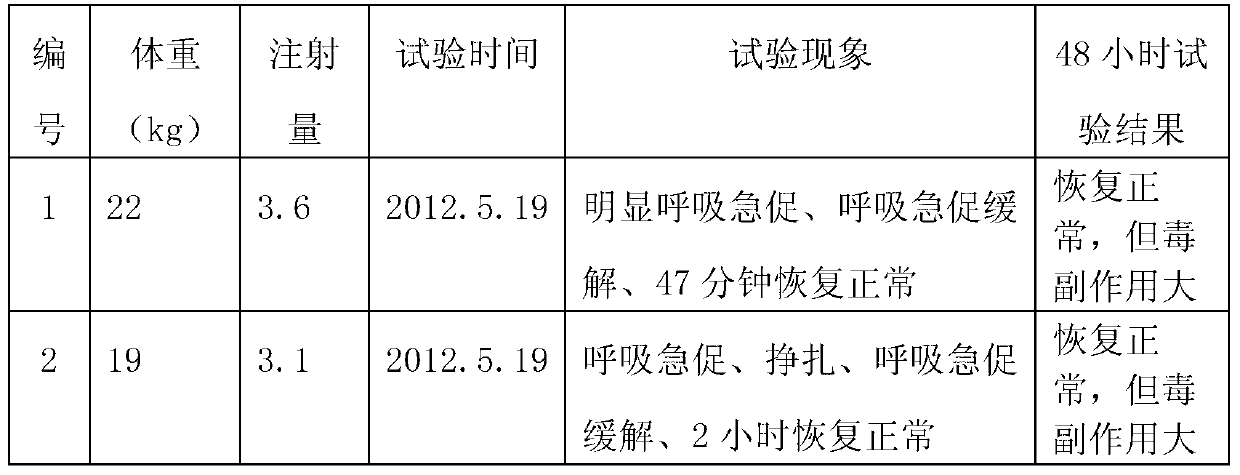
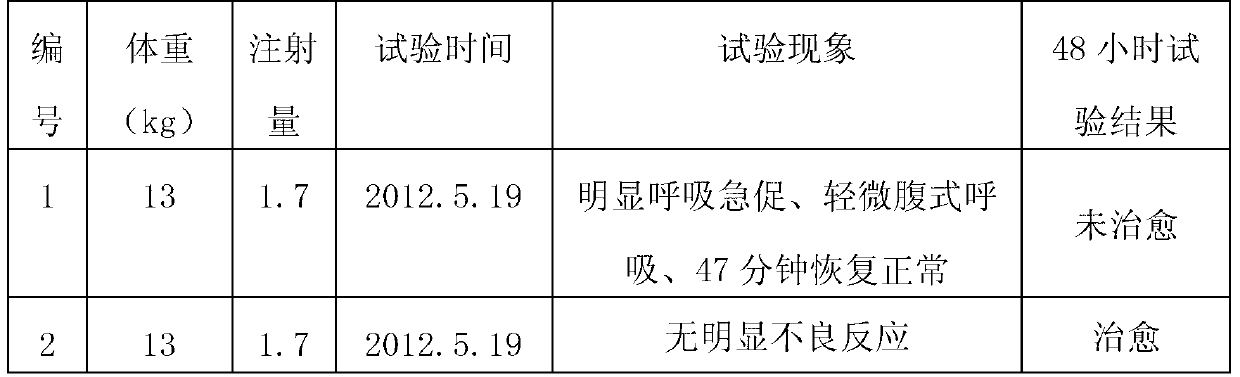
[0039] S105: Inject the first injection at 0.1ml per kilogram of body weight to pigs with porcine respiratory disease syndrome, and then inject the second injection at 0.05ml per kilogram of body weight to uncured sick pigs 48 hours later.
Embodiment 2
[0041] S201: By weight percentage, 7 parts of alcohol, 16 parts of dimethylethanolamine, 0.3 part of diaobaikuai, and 6 parts of ethanolamine.
[0042] S202: Take 30 parts of propylene glycol, add all the above components into the propylene glycol to form a mixed solution, and stir.
[0043] S203: Take 28 parts of tilmicosin, 4 parts of doxycycline, 4 parts of flunixin meglumine, and 0.03 part of dexamethasone, add them to the above mixture, and stir to make a compound tilmicosin injection.
[0044] S204: The compound tilmicosin injection is used for treating porcine respiratory syndrome.
[0045] S205: Inject the first injection at 0.08ml per kilogram of body weight to pigs suffering from porcine respiratory disease syndrome, and then inject the second injection at 0.03ml per kilogram of body weight to uncured sick pigs 48 hours later.
Embodiment 3
[0047] S301: by weight percentage, 10 parts of alcohol, 20 parts of dimethylethanolamine, 0.5 part of scalloped sage, and 5 parts of ethanolamine.
[0048] S302: Take 33 parts of propylene glycol, add all the above components into the propylene glycol to form a mixed solution, and stir.
[0049] S303: Add 30 parts of tilmicosin, 5 parts of doxycycline, 5 parts of flunixin meglumine, and 0.05 part of dexamethasone into the above mixture, and stir to make a compound tilmicosin injection.
[0050] S304: The compound tilmicosin injection is used to treat porcine respiratory syndrome.
[0051] S305: Inject the first injection of 0.11ml per kilogram of body weight to pigs with porcine respiratory disease syndrome, and then inject the second injection of 0.06ml per kilogram of body weight to uncured sick pigs 60 hours later.
[0052] The feed intake of pigs decreases during the pathogenesis of PRDC, and the dose of the drug must be increased when the feed is used to treat it. Admin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com