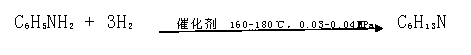Production method for preparing cyclohexylamine from phenylamine
A technology for the preparation and production of aniline, applied in the field of production and preparation of cyclohexylamine, can solve the problems of unstable control of reaction parameters, difficult recovery of catalysts, and high cost of equipment and equipment, and achieves increased product added value, large production capacity, and staff ratio. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
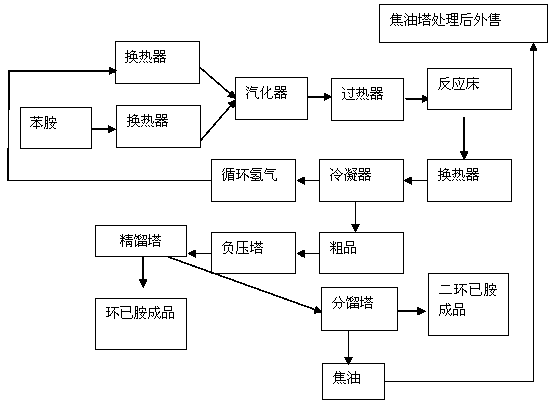
[0027] A production method for preparing cyclohexylamine from aniline. The specific steps are: the aniline is pumped to the aniline heat exchanger and preheated to 160°C, and then enters the vaporizer in the same direction as the hydrogen preheated to 140°C through the hydrogen heat exchanger, and the vaporized The temperature is 150°C, the pressure is 0.03Mpa, and the molar ratio of aniline to hydrogen is 1:15. After the aniline is completely vaporized, it enters the superheater. , in CuO / NiO / γ-Al 2 o 3 React in the presence of a catalyst, the reaction temperature is 160°C, the pressure is 0.03MPa, the reaction time is 3s-4s, and cyclohexylamine is generated; the mixed gas from the reaction bed returns to the hydrogen heat exchanger and aniline heat exchanger for heat exchange and cooling, and then enters The condenser is condensed to below 50°C, wherein the condensed liquid phase enters the crude product tank, the purity of the crude product is 96%, non-condensable gas (H ...
Embodiment 2
[0029] The production method for preparing cyclohexylamine from aniline is controlled by DCS. The specific steps are as follows: aniline is pumped to the aniline heat exchanger to be preheated to 180°C, and then enters in the same direction as the hydrogen preheated to 170°C by the hydrogen heat exchanger. Vaporizer, the vaporization temperature is 180°C, and the pressure is 0.04Mpa. The function of vaporization is to change the material from liquid to gas. The purpose is to fully contact with the catalyst and increase the contact area with the catalyst to make the reaction more complete and the conversion rate higher.
[0030] The molar ratio of aniline to hydrogen is 1:20. After the aniline is completely vaporized, it enters the superheater. The temperature of the superheater is 180°C and the pressure is 0.04Mpa. The function of the superheater is to fully vaporize the liquid.
[0031] After being heated by heat conduction oil, it enters the reaction bed, and in CuO / NiO / γ-Al ...
Embodiment 3
[0033]The production method for preparing cyclohexylamine from aniline is controlled by DCS. The specific steps are: aniline is pumped to the aniline heat exchanger to be preheated to 170°C, and then enters in the same direction as the hydrogen preheated to 160°C by the hydrogen heat exchanger. Vaporizer, the vaporization temperature is 170°C, and the pressure is 0.04Mpa. The function of vaporization is to change the material from liquid to gas. The purpose is to fully contact with the catalyst and increase the contact area with the catalyst to make the reaction more complete and the conversion rate higher.
[0034] The molar ratio of aniline to hydrogen is 1:18. After the aniline is completely vaporized, it enters the superheater. The temperature of the superheater is 170°C and the pressure is 0.035Mpa. The function of the superheater is to fully vaporize the liquid.
[0035] After being heated by heat conduction oil, it enters the reaction bed, and in CuO / NiO / γ-Al 2 o 3 Rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com