Gold nanoparticle-modified dendritic titanium dioxide nanorod array electrode, as well as preparation method and application of hydrogen production by photocatalytic water splitting
A nanorod array and gold nanoparticle technology, applied in the field of photoelectrochemistry, can solve the problems of not being able to use visible light, low photocatalytic activity, and small contact area, and achieve the effect of alleviating the shortage of fossil fuels, efficient and clean energy, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) TiO 2 Fabrication of Nanorod Arrays
[0051] ① Sonicate the FTO conductive glass in deionized water, acetone and ethanol solution for 10 minutes successively, wash it with deionized water, and dry it for later use;
[0052] ② Prepare the precursor solution of titanium source: first mix 30ml of concentrated hydrochloric acid (37.5% mass concentration) with 30ml of deionized water, stir for 5 minutes, add 1ml of butyl titanate and continue stirring for 5 minutes to obtain the titanium source precursor solution ;
[0053] ③ Place the conductive surface of the FTO conductive glass obliquely downward in the crystallization kettle, add the titanium source precursor solution, and hydrothermally synthesize it at 150°C for 14 hours to prepare TiO 2 Nanorod arrays were washed with deionized water and dried at 80°C.
[0054] (2) Dendritic TiO 2 Fabrication of Nanorod Arrays
[0055] ① Prepare 0.2M TiCl 4 Aqueous solution: the preparation process is to take a certain amou...
Embodiment 2
[0073] (1) TiO 2 The preparation of the nanorod array is the same as in Example 1.
[0074] (2) Dendritic TiO 2 The preparation of the nanorod array is the same as in Example 1.
[0075] (3) Dendritic TiO modified with Au nanoparticles 2 Fabrication of Nanorod Arrays
[0076] ① Prepare 0.9mM HAuCl 4 aqueous solution.
[0077] ② Dendritic TiO 2 The nanorod array is used as the substrate, and the method of ultraviolet light reduction (ultraviolet light intensity is 80mW / cm 2 ) to prepare Au nanoparticles modified dendritic TiO 2 Nanorod arrays with a reduction time of 9 hours.
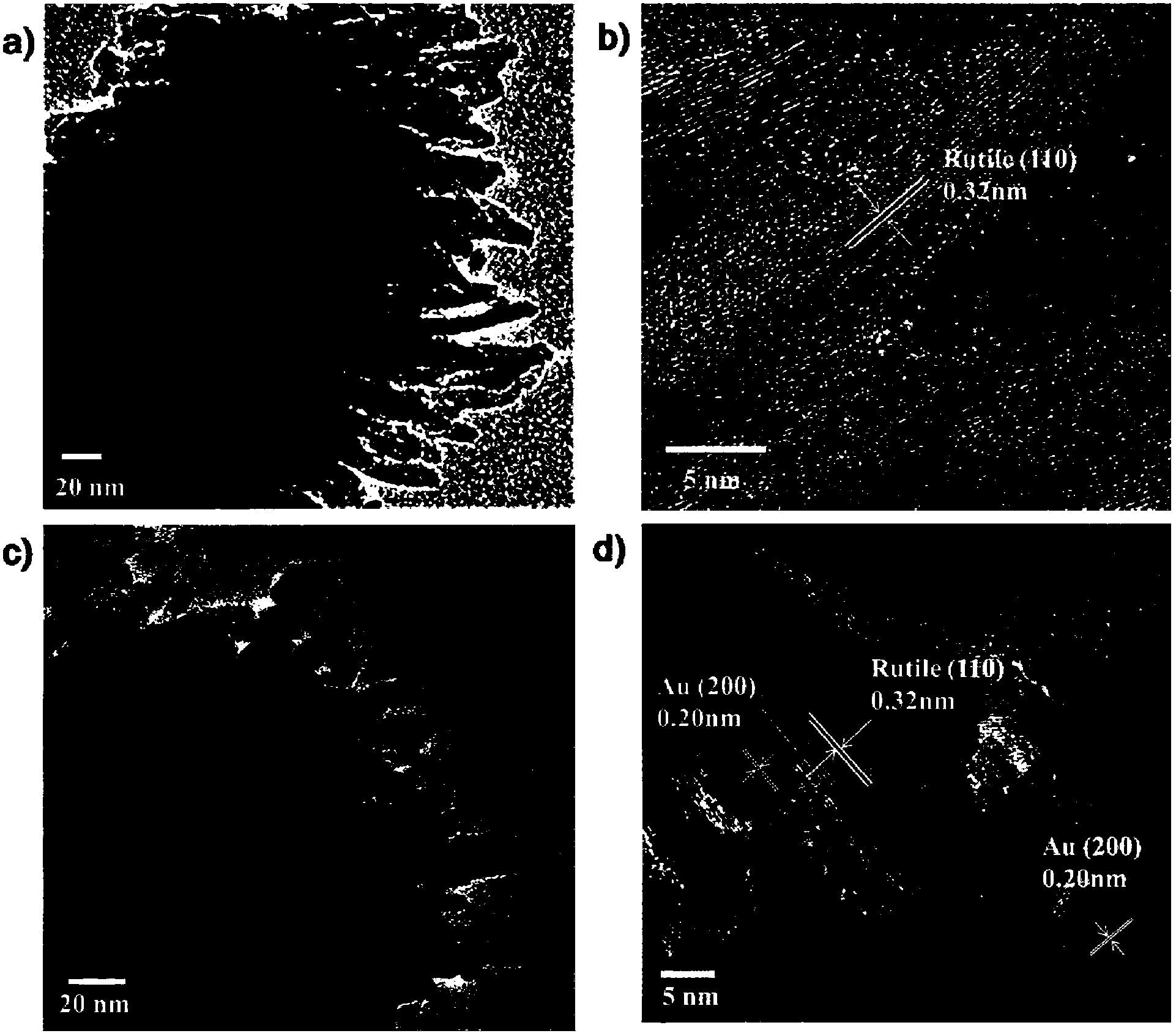
[0078] Experimental results show that the diameter of the backbone nanorods is 150-200nm and the length is 2.4μm, the diameter of the dendritic structure is 10-20nm and the length is 50-100nm, and the diameter of the Au nanoparticles is 10-20nm.
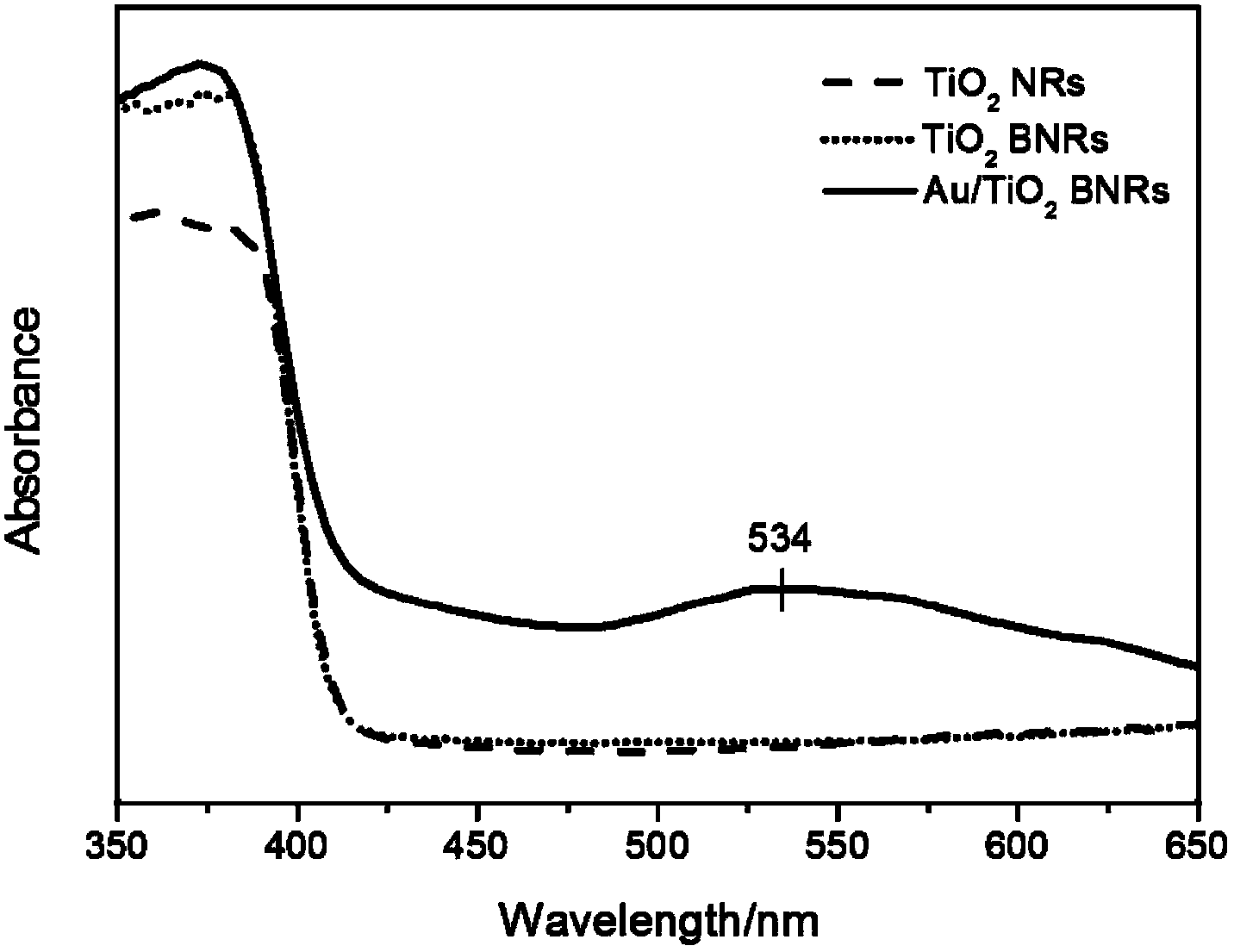
[0079] Photoelectrochemical performance tests showed that Au nanoparticles modified dendritic TiO 2 The photocurrent density of nanorod array is 2.0m...
Embodiment 3
[0081] (1) TiO 2 The preparation of the nanorod array is the same as in Example 1.
[0082] (2) Dendritic TiO 2 Fabrication of Nanorod Arrays
[0083] ① Prepare 0.1M TiCl 4 Aqueous solution: the preparation process is to take a certain amount of TiCl under stirring conditions 4 soluble in ice water;
[0084] ②The prepared TiO 2 Nanorod arrays placed in 0.2M TiCl 4 Amorphous dendritic TiO was prepared by chemical water bath deposition under sealed conditions in aqueous solution for 24 hours 2 nanorod arrays;
[0085] ③ The above-prepared amorphous dendritic TiO 2 Nanorod arrays were fired under air atmosphere for 30 min to form dendritic TiO 2 nanorod arrays.
[0086] (3) Dendritic TiO modified with Au nanoparticles 2 Fabrication of Nanorod Arrays
[0087] ① Prepare 1.5mM HAuCl 4 aqueous solution.
[0088] ② Dendritic TiO 2 The nanorod array is used as the substrate, and the method of ultraviolet light reduction (ultraviolet light intensity is 120mW / cm 2 ) to pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com