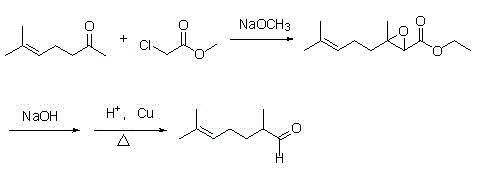
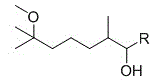
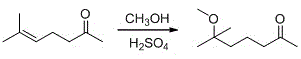2,6-dimethyl-6-methoxy heptanol series derivatives and preparation method thereof
A technology of methoxyheptanol and methoxyheptaldehyde, which is applied in the field of 2,6-dimethyl-6-methoxyheptanol series derivatives and the preparation thereof, can solve the problems of inconvenient use, melon aldehyde fragrance Instability and other problems, to achieve the effect of stable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] A 2,6-dimethyl-6-methoxyheptanol series derivative, wherein R is ethyl.
[0064] The above-mentioned preparation method of a kind of 2,6-dimethyl-6-methoxyheptanol series derivatives specifically comprises the following steps:
[0065] (1) Alkylation reaction
[0066] Add 440 grams of methyl heptenone and 1100 grams of methanol into a 2000 mL four-neck flask, add 65 grams of 95% concentrated sulfuric acid under stirring, heat to 50 ° C, reflux for 22 hours, GC analysis, the reaction product conversion is 83.5%, add sodium carbonate 70.4g, neutralize sulfuric acid, recover methanol, add 500g water to dissolve inorganic substances, layer the system, separate liquids, add methyl tert-butyl ether (150ml*3) to the water layer for extraction, combine organic layers, and distill under reduced pressure to obtain the product 319.3 grams of 6-methyl-6-methoxy-2-heptanone, yield 58%, GC content 91.2%;
[0067] (2), Darzens condensation reaction
[0068] Take 0.2mol (31.6g) of 6...
Embodiment 2
[0104] A 2,6-dimethyl-6-methoxyheptanol series derivative, wherein R is cyclohexyl.
[0105] (1), alkylation reaction is the same as embodiment 1;
[0106] (2), Darzens condensation reaction is the same as embodiment 1;
[0107] (3) Addition reaction of 2,6-dimethyl-6-methoxyheptanal and cyclohexylmagnesium bromide
[0108] Add 0.0165mol (0.4g) of dried magnesium flakes into the flask, immerse in tetrahydrofuran, add 1 to 2 drops of bromocyclohexane and 1 grain of iodine, and heat to initiate the reaction, and 0.015mol (2.5g) of brominated The mixed solution obtained by dissolving cyclohexane in 15g tetrahydrofuran is added dropwise at a rate of 0.4-0.8ml / min, and reacted for about 1 hour after the dropwise addition, and cooled to -40°C in a low-temperature reaction bath, and then the step ( 2) The resulting 0.01mol (1.7g) 2,6-dimethyl-6-methoxyheptanal was dissolved in 10g tetrahydrofuran to obtain a solution of 2,6-dimethyl-6-methoxyheptanal dropwise Add it at an accelera...
Embodiment 3
[0122] A 2,6-dimethyl-6-methoxyheptanol series derivative, wherein R is allyl.
[0123] (1), alkylation reaction is the same as embodiment 1;
[0124] (2), Darzens condensation reaction is the same as embodiment 1;
[0125] (3) Addition reaction of 2,6-dimethyl-6-methoxyheptanal and allylmagnesium bromide
[0126] Add 0.0165mol (0.4g) of dried magnesium flakes into the flask, immerse in tetrahydrofuran, add 1-2 drops of allyl bromide and 1 grain of iodine, after the reaction is initiated, dissolve 0.3g of allyl bromide in 5g of tetrahydrofuran The obtained mixed solution is added dropwise at a controlled rate of 0.4-0.8ml / min. After the dropwise addition, the reaction temperature is maintained at 10-20°C, and then 0.01mol (1.7g) 2 , 2,6-dimethyl-6-methoxyheptanal solution obtained by dissolving 6-dimethyl-6-methoxyheptanal in 10 g tetrahydrofuran, and consisting of 1.4 g allyl bromide and 20 g tetrahydrofuran The mixed solution was added at a rate of 0.4 to 0.8ml / min. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com