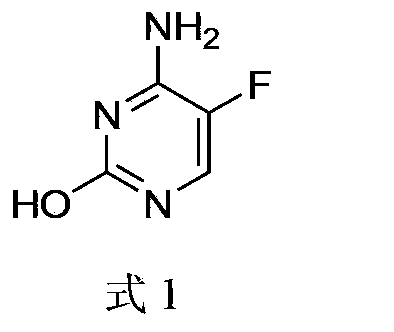
5-fluorocytosine preparation method
A technology of fluorocytosine and methyl fluoroacetate, which is applied in the field of preparation of 5-fluorocytosine, can solve problems such as high cost, complex equipment, and large pollution, and achieve reduced production cycle and cost, easy recycling, and reduced waste liquid The effect of emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
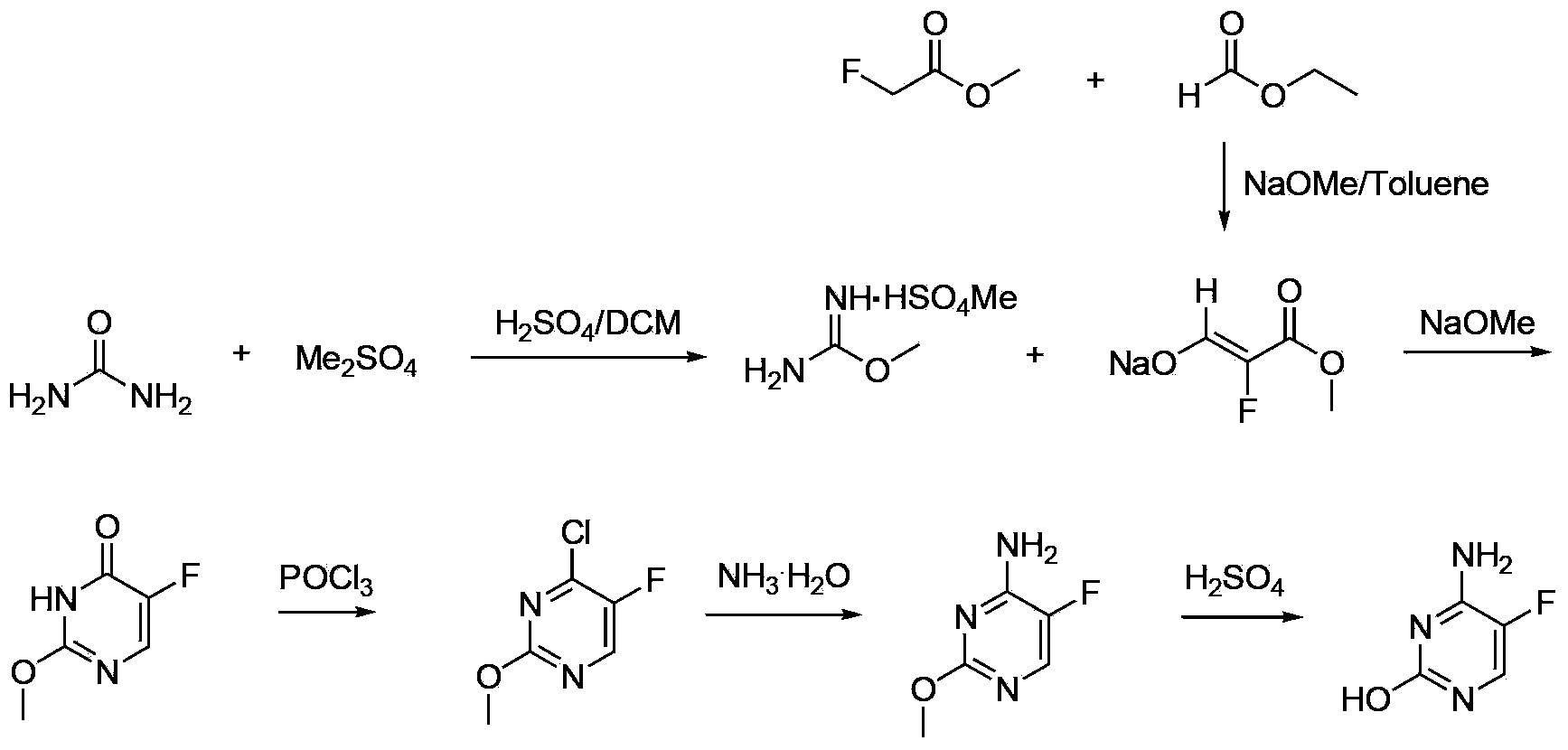
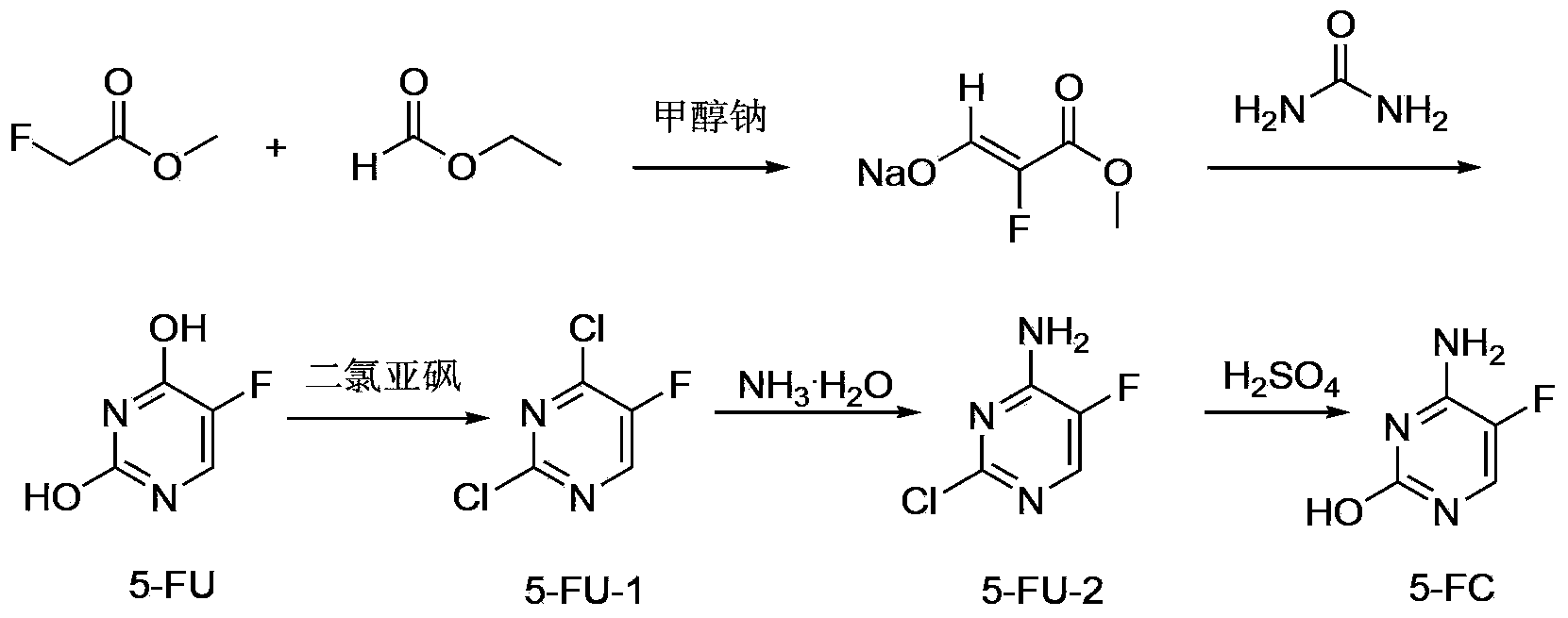
[0032] The specific operation steps of the preparation method of 5-fluorocytosine include:
[0033] a. Add sodium methoxide to toluene in batches. After the addition, first add ethyl formate dropwise, then add methyl fluoroacetate dropwise. After the dropwise stirring is completed for 1.0-2.0 hours, heat up to 36-38°C for 5-8 hours; Then add methanol and sodium methoxide, stir and lower the temperature to 15-25°C, then add urea to react for 4-6 hours. Adjust the pH to 3-4 with hydrochloric acid, control the temperature below 20°C and stir for 1-2 hours; filter to obtain a solid, wash with water to obtain compound 5-FU;
[0034] b. 5-FU, thionyl chloride and DMF are heated up to reflux reaction until the content of 5-FU is ≤0.2%. After the reaction is over, atmospheric distillation recovers thionyl chloride, and then distillation under reduced pressure yields compound 5- FU-1; Wherein, DMF is as the catalyst of chlorination reaction;
[0035] c. Heat ammonia water and 5-FU-1 ...
Embodiment 1
[0056] a. In a 1L three-necked flask, nitrogen gas was introduced, and toluene (500 g) was added. Sodium methoxide (52.9 g, 0.98 mol) was added in batches, and the temperature was controlled below 30°C. After the addition, the temperature was lowered to 10°C, and ethyl formate (72.5 g, 0.98 mol) was added dropwise. During the dropwise addition, the temperature of the reaction system was controlled at 10-20°C.
[0057] After the ethyl formate was added dropwise, methyl fluoroacetate (60 g, 0.65 mol) was added dropwise, and the temperature was controlled at 15-20°C during the dropwise addition. After dripping and stirring for 1.0 h, the temperature was raised to 36-38° C., and the reaction was stirred for 5 h. Add methanol (65g) and sodium methoxide (30g, 0.56mol), stir and cool down to 20°C.
[0058] Urea (67.2 g, 1.12 mol) was added, and the reaction was stirred at room temperature (28° C.) for 5 hours. Recover methanol by desolvation under reduced pressure, and then add wa...
Embodiment 2
[0063] a. In a 1L three-necked flask, nitrogen gas was introduced, and toluene (500 g) was added. Sodium methoxide (52.9 g, 0.98 mol) was added in batches, and the temperature was controlled below 30°C. After the addition, the temperature was lowered to 10°C, and ethyl formate (72.5 g, 0.98 mol) was added dropwise. During the dropwise addition, the temperature of the reaction system was controlled at 10-20°C.
[0064] After the ethyl formate was added dropwise, methyl fluoroacetate (60 g, 0.65 mol) was added dropwise, and the temperature was controlled at 15-20°C during the dropwise addition. After dripping and stirring for 1.0 h, the temperature was raised to 36-38° C., and the reaction was stirred for 5 h. Add methanol (65g) and sodium methoxide (30g, 0.56mol), stir and cool down to 20°C.
[0065] Urea (67.2 g, 1.12 mol) was added, and the reaction was stirred at room temperature (28° C.) for 5 hours. Recover methanol by desolvation under reduced pressure, and then add wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com