Stable and efficient wet oxidation catalyst and preparation method thereof
A wet oxidation and catalyst technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve problems such as catalyst deactivation, achieve simple operation and improve utilization. Efficient, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
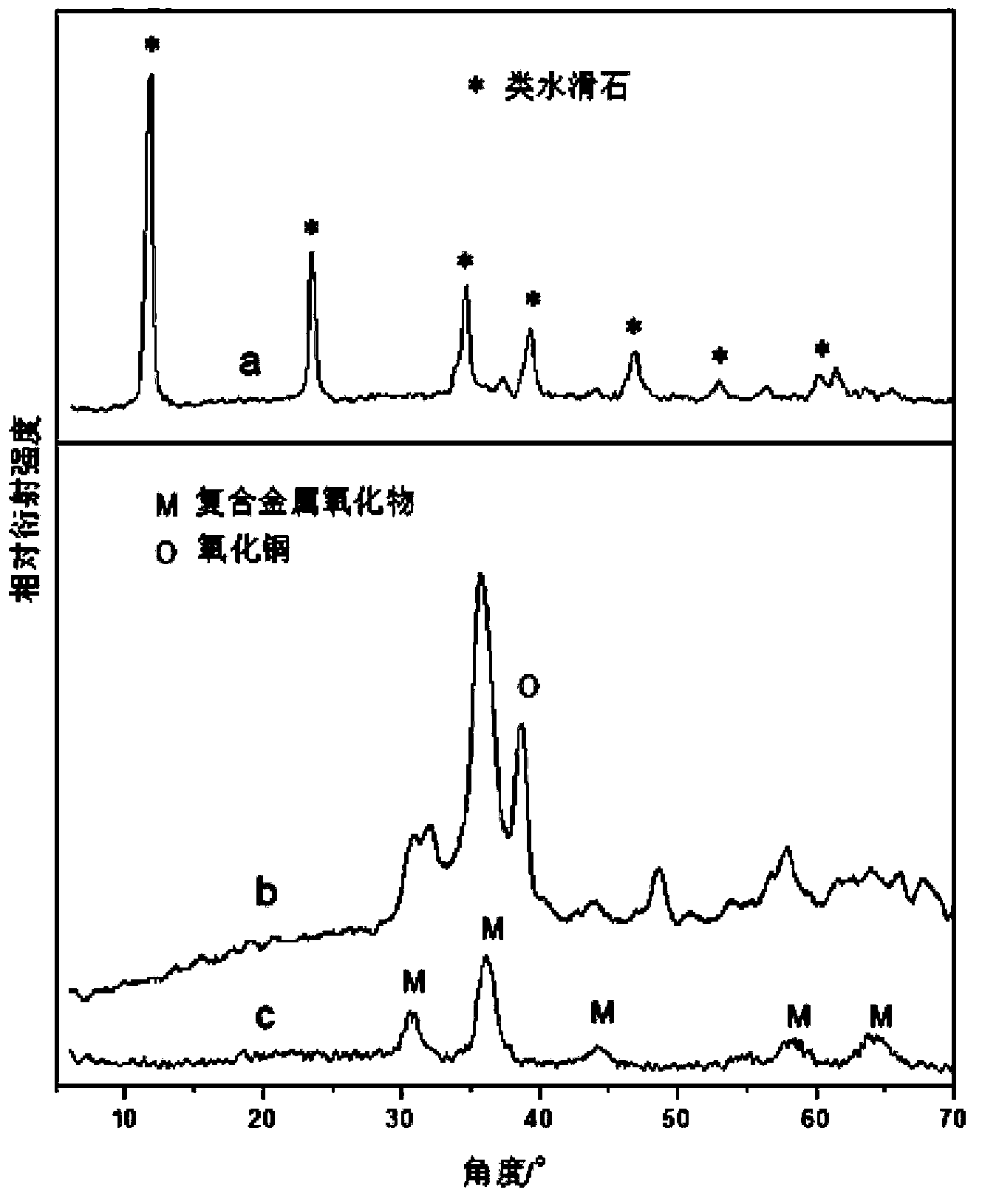
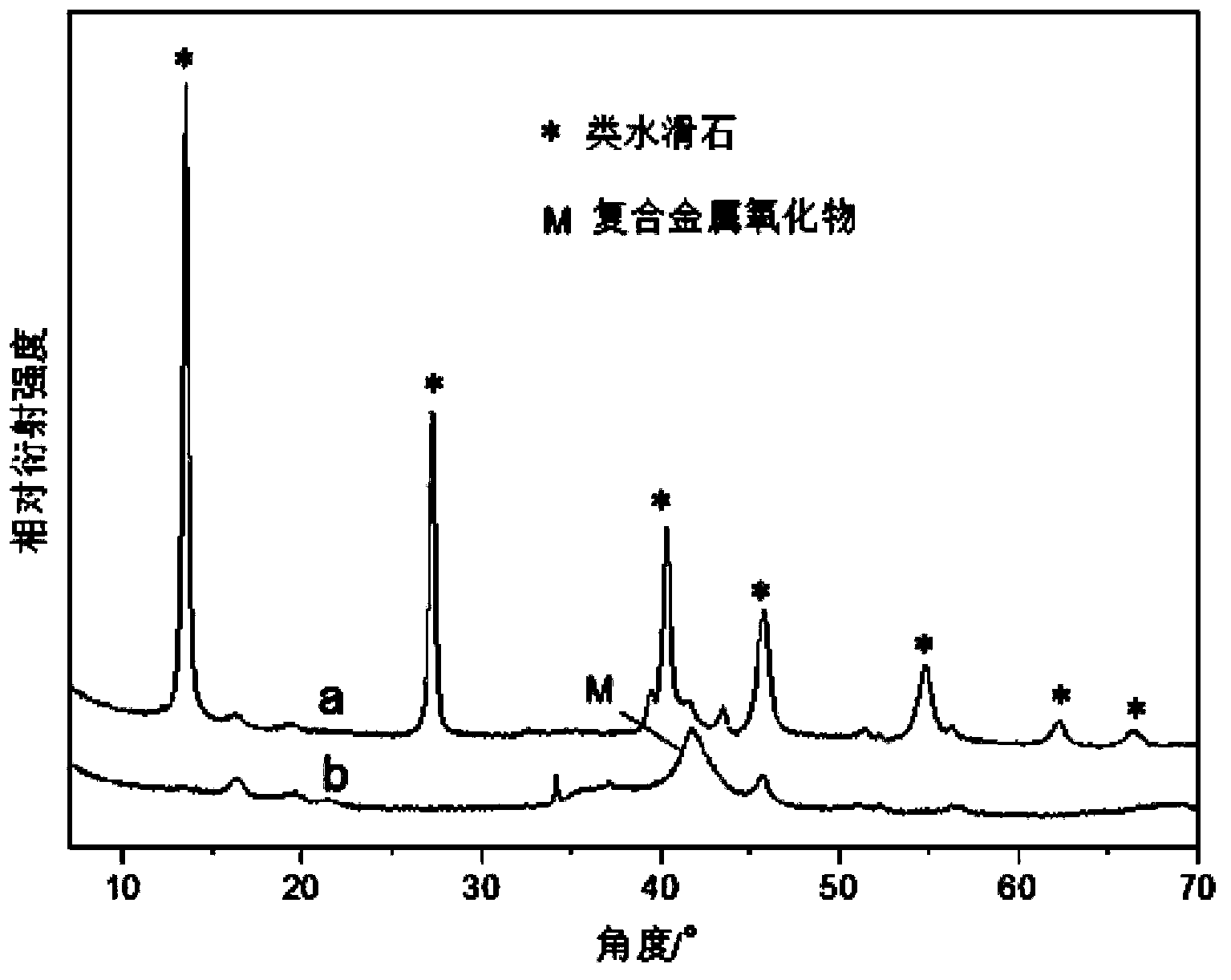
[0026]Dissolve 8.46g of copper nitrate, 9.52g of zinc nitrate, 9.38g of aluminum nitrate, 3.23g of ferric nitrate and 0.15g of lanthanum nitrate in 100ml of water to prepare a mixed salt solution. Dissolve 11.20g of potassium hydroxide and 6.30g of sodium carbonate in 100ml of water to prepare alkali solution and carbonate solution. Add the mixed salt solution and potassium hydroxide solution slowly and dropwise to the vigorously stirred sodium carbonate solution at the same time, control the drop rate of the salt and alkali, and keep the pH value of the solution at 10±0.2. After the mixed salt solution is consumed, stop the dropwise addition. The precipitate was stirred and crystallized in a water bath at 60°C for 24 hours, the obtained slurry was filtered and washed until neutral, and the filter cake was dried overnight in an oven at 60°C. figure 1 In a, it can be seen that the obtained powder is hydrotalcite-like, and the precursor powder is placed in a muffle furnace at 2°...
Embodiment 2
[0030] Dissolve 9.38g of copper nitrate, 14.87g of zinc nitrate, 13.13g of aluminum nitrate, 5.05g of ferric nitrate and 1.08g of lanthanum nitrate in 150ml of water to prepare a mixed salt solution. Dissolve 16.20g of sodium hydroxide and 5.30g of sodium carbonate in 100ml of water to prepare alkali solution and carbonate solution. Slowly add the mixed salt solution and sodium hydroxide solution dropwise into the vigorously stirred sodium carbonate solution at the same time, control the drop rate of salt and alkali, and keep the pH value of the solution at 10±0.2. After the mixed salt solution is consumed, stop the dropwise addition. The precipitate was stirred and crystallized in a water bath at 60°C for 48 hours, the obtained slurry was filtered and washed until neutral, and the filter cake was dried overnight in an oven at 60°C. figure 2 It can be seen in a that the obtained powder is hydrotalcite-like, and the precursor powder is placed in a muffle furnace at 5°C·min -1...
Embodiment 3
[0033] Dissolve 6.25g of copper nitrate, 6.11g of zinc acetate, 7.99g of aluminum sulfate, 2.00g of ferric sulfate and 2.17g of lanthanum nitrate in 100ml of water to prepare a mixed salt solution. Prepare 100ml of 7.0% dilute ammonia water, and dissolve 4.80g of ammonium carbonate in 100ml of water to prepare alkali solution and carbonate solution. Add the mixed salt solution and dilute ammonia water slowly and dropwise to the vigorously stirred ammonium carbonate solution at the same time, control the drop rate of salt and alkali, and keep the pH value of the solution at 10±0.2. Stir and crystallize the material in a water bath at 60°C for 20 hours, filter and wash the obtained slurry to neutrality, and dry the filter cake in an oven at 80°C overnight, then place the precursor powder in a muffle furnace at 10°C min -1 The heating rate was raised from room temperature to 650°C and roasted for 2 hours to obtain the roasted product, which was filtered and washed with 0.5 mol / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com