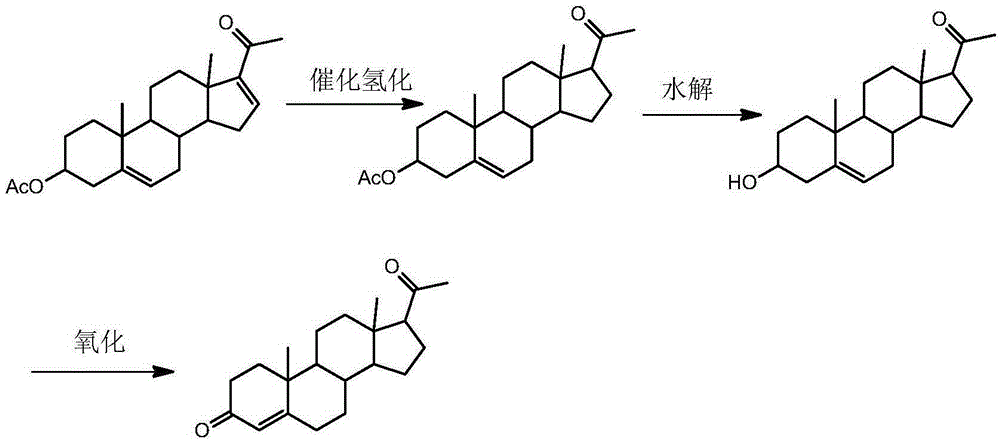
A kind of synthetic method of pregnenolone acetate
A technology of pregnenolone acetate and dienolone acetate, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of small output and large amount of solvent, and achieve the effects of low cost, high selectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step 1. Add 750L of ethyl acetate, 100kg of dienolone acetate and 5kg of ammonium acetate to a clean 1000L hydrogenation reactor, and suck in the ethyl acetate mixed solution of palladium / carbon catalyst (0.5kg loading capacity 5 kg) under nitrogen protection. % palladium / carbon catalyst into 50L ethyl acetate), nitrogen replacement 3 times, hydrogen replacement 3 times; the temperature of the reaction kettle was raised to 45°C, the hydrogen pressure was kept at 0.4MPa, and the reaction was stirred for 2 hours, and no ultraviolet absorption was detected by TLC;
[0027] Step 2, filter the reaction solution after the reaction in step 1, transfer the filtered filtrate to the concentration kettle, concentrate and reclaim ethyl acetate at normal pressure until the concentration kettle wall has crystals to separate out, then add 50L mixed solvent in the concentration kettle, The temperature is lowered to below 5° C., and then centrifuged to dry to obtain the crude product of ...
Embodiment 2
[0031] Step 1. Add 850L of ethyl acetate, 100kg of dienolone acetate and 6kg of ammonium acetate to a clean 1000L hydrogenation reactor, and suck in the ethyl acetate mixture of palladium / carbon catalyst (1kg loading capacity 5% The palladium / charcoal catalyst was added to 50L ethyl acetate), nitrogen replacement 5 times, hydrogen replacement 5 times; the temperature of the reaction kettle was raised to 40°C, the hydrogen pressure was kept at 0.6MPa, and the reaction was stirred for 2.5h, and no ultraviolet absorption was detected by TLC;
[0032] Step 2, filter the reaction solution after the reaction in step 1, transfer the filtered filtrate to the concentrated kettle, concentrate and reclaim ethyl acetate at normal pressure until the wall of the concentrated kettle still has crystals, then add 100L mixed solvent in the concentrated kettle, The temperature is lowered to below 5° C., and then centrifuged to dry to obtain the crude product of pregnenolone acetate; the mixed sol...
Embodiment 3
[0036] Step 1. Add 950L of ethyl acetate, 100kg of dienolone acetate and 7kg of ammonium acetate to a clean 1000L hydrogenation reactor, and suck in the ethyl acetate mixed solution of palladium / carbon catalyst (1.5kg loading capacity 5 kg) under nitrogen protection. % palladium / charcoal catalyst into 50L ethyl acetate), nitrogen replacement 4 times, hydrogen replacement 4 times; the reaction kettle was heated to 35°C, the hydrogen pressure was kept at 0.8MPa, and the reaction was stirred for 3 hours, and no ultraviolet absorption was detected by TLC;
[0037] Step 2, filter the reaction solution after the reaction in step 1, transfer the filtered filtrate to the concentrated kettle, concentrate and reclaim ethyl acetate at normal pressure until the wall of the concentrated kettle has crystals to separate out, then add 150L mixed solvent in the concentrated kettle, The temperature is lowered to below 5° C., and then centrifuged to dry to obtain the crude product of pregnenolone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com