A kind of poly(aryl ether ketone) and preparation method thereof
A technology of polyaryl ketone and degree of polymerization, which is applied in the field of polyarylether ketone and its preparation, can solve the problems of material modulus drop, not high, difficult processing, etc., achieve good dimensional stability, improve heat resistance, improve resistance thermal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention also provides a preparation method of polyaryletherketone represented by the above formula (I), comprising:
[0039] A) Under the protection of inert gas, mix the imidazole monomer, the disubstituted benzophenone with the structure of formula (III) and the first aprotic solvent, and heat the reaction under the action of carbonate to obtain the structure of formula (I) The polyaryletherketone, the imidazole monomer has a structure of formula (II-1) or formula (II-2);
[0040]
[0041] The -Ar- is selected from one of the following structures of formula (1) to formula (2):
[0042]
[0043] Among them, A 1 with A 2 Each independently is S or O; R 1 with R 2 each independently selected from H, NH 2 , NO 2 One of C1~C5 alkyl groups; R 3with R 4 Each independently selected from one of H, phenyl and substituted phenyl; n is a polymer; X is a halogen atom or NO 2 , preferably Cl, F or NO 2 . The R 1 , R 2 , R 3 with R 4 All are the sa...
Embodiment 1
[0058] Add 1.341g (0.01mol) of benzimidazolone (HBI), 2.182g (0.01mol) of difluorobenzophenone (DFK), 2.763g of potassium carbonate, 25g of sulfolane and 12ml of toluene in a 100ml three-neck round bottom flask, Heat to 130°C to azeotropically remove water, keep warm for 2 hours, raise the temperature to remove toluene, continue to heat to 185°C, react for 4.5 hours, cool down, add 20ml dimethylacetamide to dilute, and precipitate in the mixed solution of ethanol, water and acetic acid, precipitate After filtration, extract with deionized water in a Soxhlet extractor for 12 hours, remove inorganic salts and reaction solvents, and dry to obtain polyaryletherketone represented by formula (I-a), whose reaction formula is as follows:
[0059]
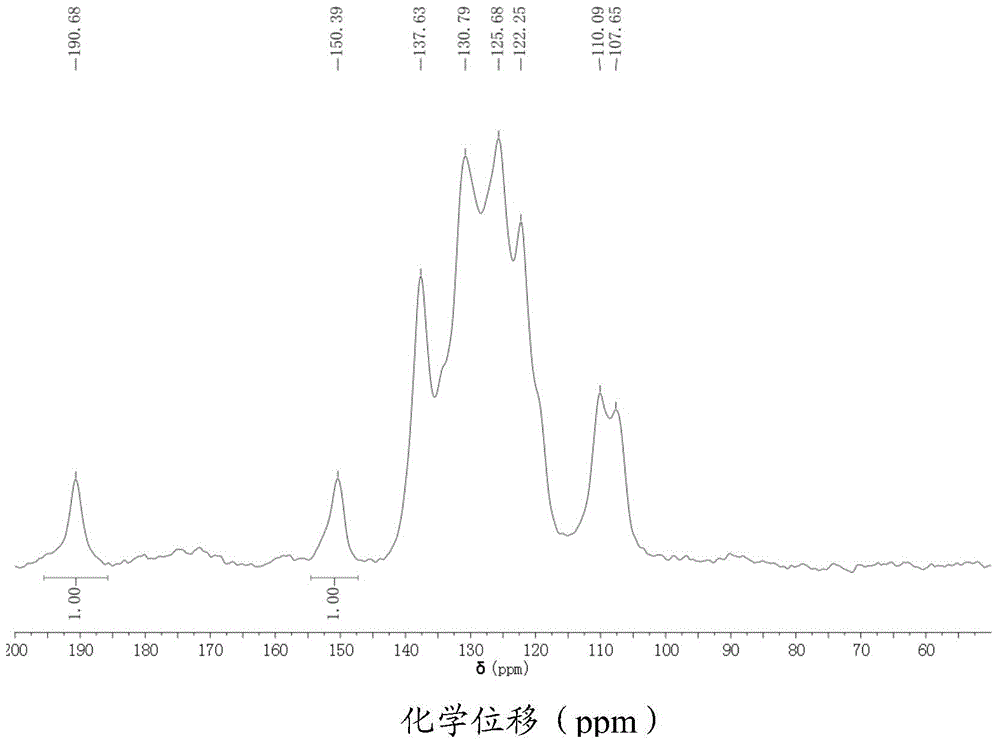
[0060] The polyaryletherketone shown in the formula (I-a) obtained in Example 1 is tested by nuclear magnetic resonance, and its solid nuclear magnetic quantitative carbon spectrum is obtained, as shown in figure 1 shown. Depend on fig...
Embodiment 2
[0064] Add 1.482g (0.01mol) 5-methylbenzimidazolone (mHBI), 2.182g (0.01mol) difluorobenzophenone (DFK), 1.590g potassium carbonate, 12.5g N-cyclohexylpyrrolidone (CHP) and 12ml toluene, heated to 150°C, kept warm for 2h, azeotropically removed water, raised temperature to remove toluene, continued heating to 190°C, reacted for 4.5h, then heated to 205°C, reacted for 2h, cooled down, Add 10ml of dimethylacetamide for dilution, precipitate in a mixed solution of ethanol, water and hydrochloric acid, filter the precipitate, extract it with deionized water for 12 hours in a Soxhlet extractor, remove inorganic salts and reaction solvents, and dry to obtain the formula The polyaryletherketone shown in (I-b) has the following reaction formula:
[0065]
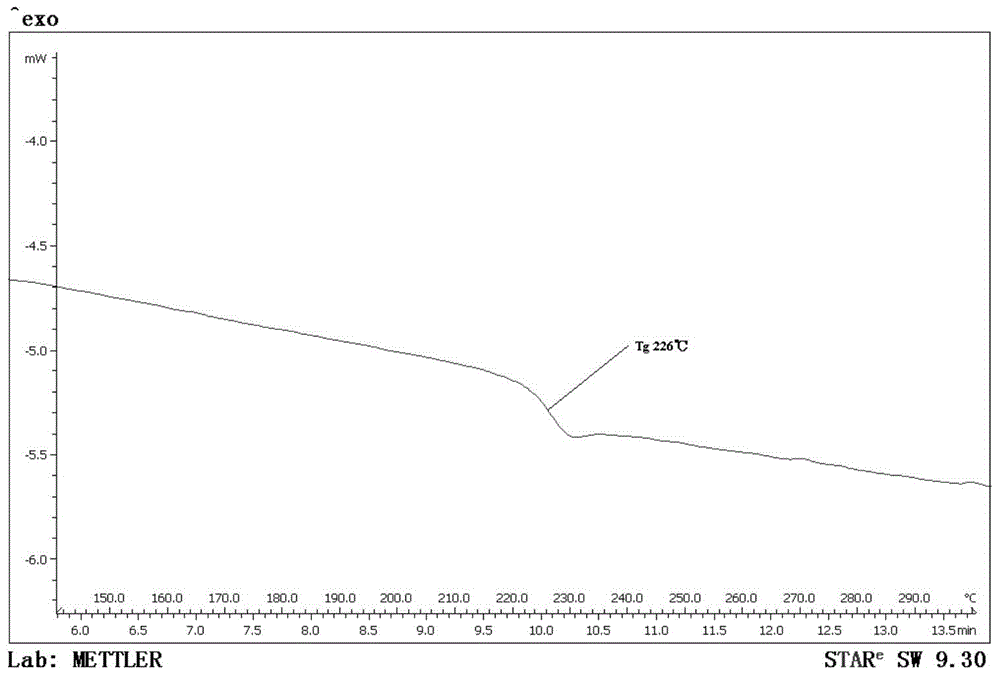
[0066] The polyaryletherketone represented by the formula (I-b) obtained in Example 2 was tested for its glass transition temperature, and its Tg was found to be 235°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com