Application of human embryonic lung fibroblast strains in preparation of rabies inactivated vaccine
A fibroblast and inactivated vaccine technology, applied in the application field of human embryonic lung fibroblast cell line in the preparation of rabies inactivated vaccine, can solve the problem of inability to guarantee cell matrix tumorigenesis and cell DNA, difficult to cultivate cells, and difficult to spread Universal use and other issues, to achieve clear property rights of genetic resource stakeholders, clear genetic background information, and facilitate the production scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of embodiment 1 Walvax-2 cell strain
[0036]1.1. Acquisition of experimental materials: After obtaining the approval of the national health management department and the ethics committee, investigate the age, occupation and health status of the couples who donate pregnant women, as well as the three generations of parents of the fetus. Fetuses with a family history of genetic defects. After obtaining the consent of the parents of the fetus and their relatives, sign the "Donor Consent".
[0037] 1.2. Primary culture: embryos sent to the laboratory in time after induction of water sac, the lung tissue was taken out under sterile conditions, the membrane on the surface of the lung tissue was torn off, and cut into 1-2 mm 3 The tissue pieces were washed with PBS for 3 to 4 times; the tissue pieces were transferred into the Erlenmeyer flask; digested with trypsin for 30 minutes, neutralized with serum, centrifuged at 1000rpm for 5 minutes to discard the trypsin...
Embodiment 2
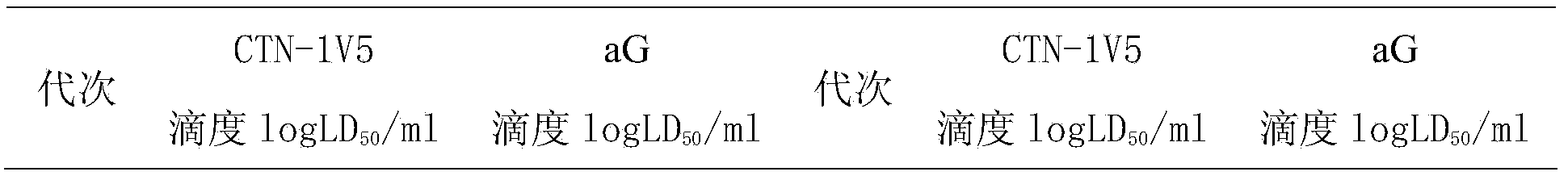
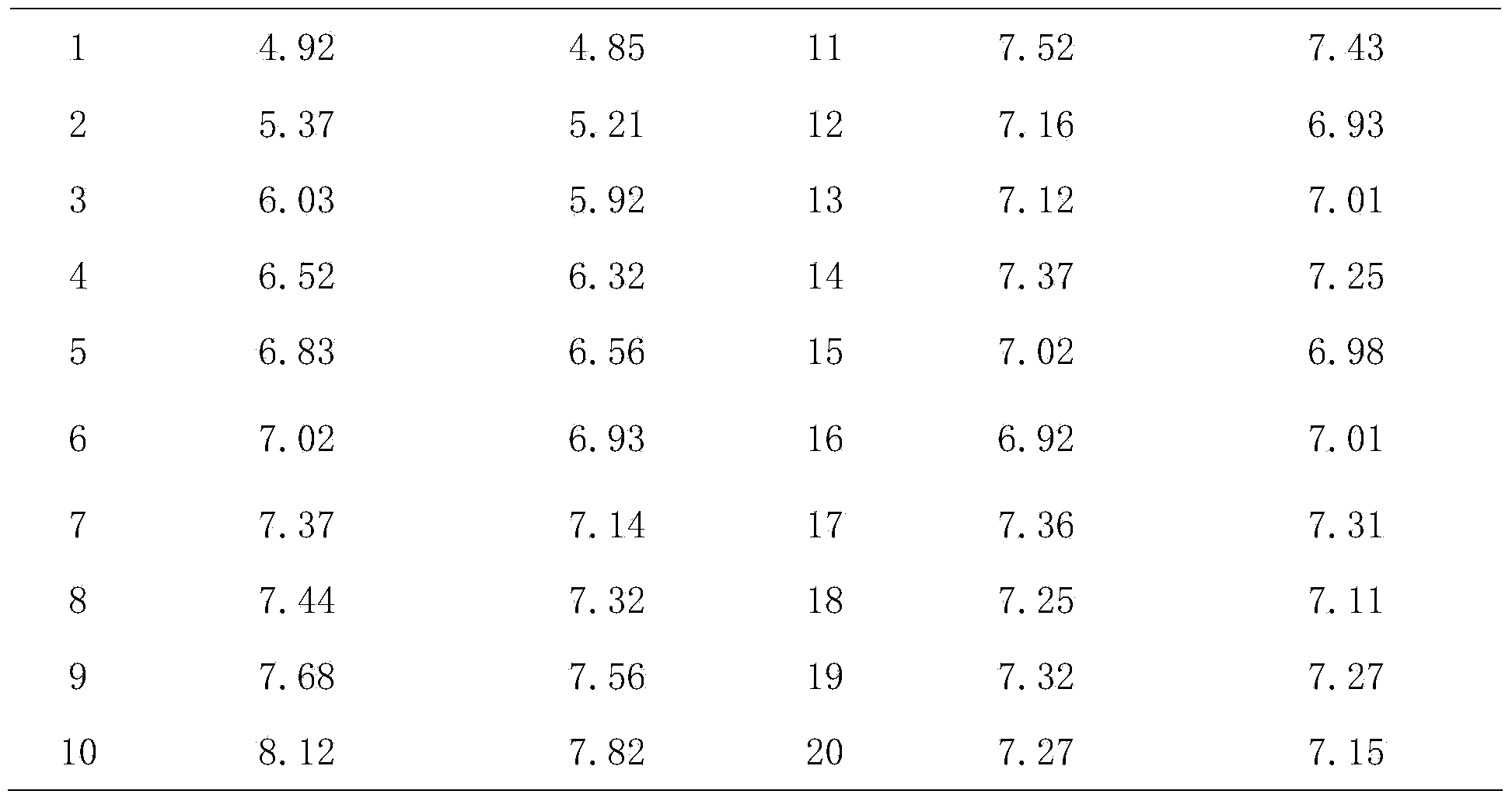
[0043] Embodiment 2 Adaptation of rabies virus CTN-1V5 virus and aG virus on cell Walvax-2 respectively
[0044] 2.1 Resuscitate Walvax-2 cells by conventional methods, use MEM containing 10% calf serum to subculture to 25-30 passages, grow into a monolayer, digest, disperse into single cells, add complete culture medium, centrifuge and remove the supernatant, the cells Suspending the sediment with serum-free medium to obtain a suspension of human embryonic lung diploid cells dispersed as single cells;
[0045] 2.2 Inoculate the fresh rabies virus CTN-1V5 seed into the human embryonic lung diploid cell suspension dispersed into single cells at 0.0001-1 MOI, absorb at 36°C for 90 minutes, and add cell culture medium to a suitable volume of 8 ml , cultivate at 30-40°C, form a dense monolayer in 2-5 days, discard the cell culture medium, replace with MEM maintenance solution containing 5% calf serum and 1% sucrose solution, maintain for 5 days, harvest the supernatant, and add 5-...
Embodiment 3
[0055] The rabies inactivated vaccine effect comparison prepared by embodiment 3 Walvax-2 cells and MRC-5 cell lines
[0056] The rabies inactivated vaccine prepared by Walvax-2 cell line is compared with the vaccine effect prepared by MRC-5 cells, and the method of Example 1 is used to prepare rabies inactivated vaccine on MRC-5 cells, and there are the following differences.
[0057] Table 2: Comparison of the effects of inactivated rabies vaccines prepared by Walvax-2 cells and MRC-5 cell lines
[0058] difference
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com