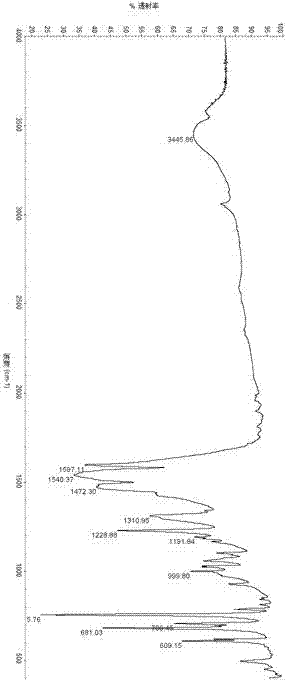
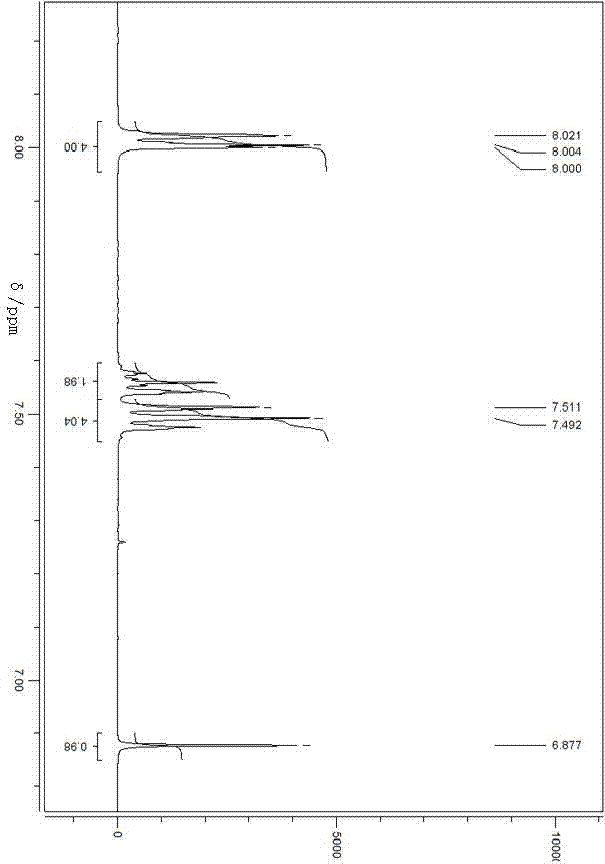
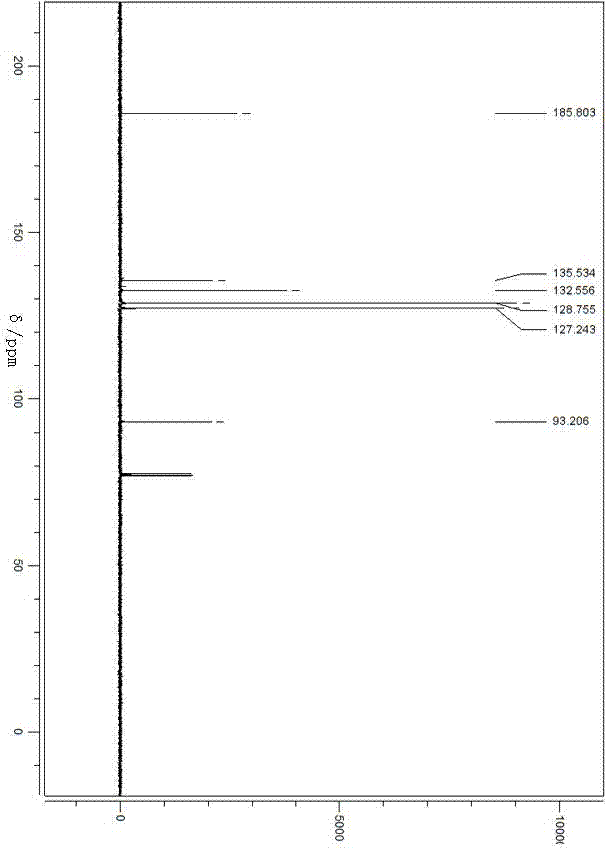
Preparation for dibenzoyl methane
A technology of dibenzoylmethane and methyl benzoate, which is applied in the field of sodium hydride to catalyze the preparation of dibenzoylmethane, can solve the problems of reduced catalyst activity, high reaction temperature, and increased by-products, so as to reduce industrial energy consumption, The effect of lowering the reaction temperature and increasing the product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 62mL of toluene into a 250mL three-neck flask, stir it mechanically, and all the instruments used need to be dried in advance. Under the protection of nitrogen, add 2.0g of sodium hydride and 11.3g of methyl benzoate in sequence, keep the temperature of the system at 0°C, start to add 10g of acetophenone dropwise, after 0.5h the dropwise addition is completed, then raise the reaction temperature to 50°C and stir After 0.5h, the hydrogen generated by the system was discharged through the bubbler. When no hydrogen gas is produced, the nitrogen flow is stopped. Add 60mL of 15% (mass fraction) hydrochloric acid to the reaction solution for acidification, increase the stirring force until the viscous substance completely turns into liquid, after the viscous substance disappears, separate the water phase, and pour the organic phase into the liquid separation In the funnel, use 5% (mass fraction) NaHCO 3 Wash with 60mL of alkali, remove the water phase, wash the organic ...
Embodiment 2
[0022] Add 70mL of toluene into a 250mL three-neck flask, stir it mechanically, and all the instruments used need to be dried in advance. Under the protection of nitrogen, add 3.6g of sodium hydride and 22.6g of methyl benzoate in sequence, keep the temperature of the system at 30°C, start to add 10g of acetophenone dropwise, after 1h, stir for 1h, and the hydrogen generated by the system passes through the drum The bubbler is excluded. When no hydrogen gas is produced, the nitrogen flow is stopped. Add 30mL of 50% (mass fraction) hydrochloric acid to the reaction solution for acidification, and increase the stirring force until the viscous substance completely turns into a liquid. After the viscous substance disappears, separate the water phase, and then use 30% ( mass fraction) NaHCO 3 Wash with 40mL of alkali, then wash with water, remove the solvent and excess methyl benzoate after vacuum distillation to obtain the light yellow crude product, heat and dissolve the crude ...
Embodiment 3
[0024] Add 620mL of toluene into a 1L three-neck flask, stir it mechanically, and all the instruments used need to be dried in advance. Under the protection of nitrogen, add 13.9g of sodium hydride and 85.1g of methyl benzoate in sequence, keep the temperature of the system at 0°C, start to add 50g of acetophenone dropwise, the dropwise addition is completed after 2h, then raise the reaction temperature to 40°C and stir for 1h , the hydrogen produced by the system is removed through the bubbler. When no hydrogen gas is produced, the nitrogen flow is stopped. Add 150mL of 30% (mass fraction) hydrochloric acid to the reaction liquid for acidification, increase the stirring force until the viscous substance becomes liquid completely, after the viscous substance disappears, separate the water phase, and use 10% (mass fraction) for the organic phase fraction) NaHCO 3 Wash with 150mL of alkali, then wash with water, remove the solvent and excess methyl benzoate after distillation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com