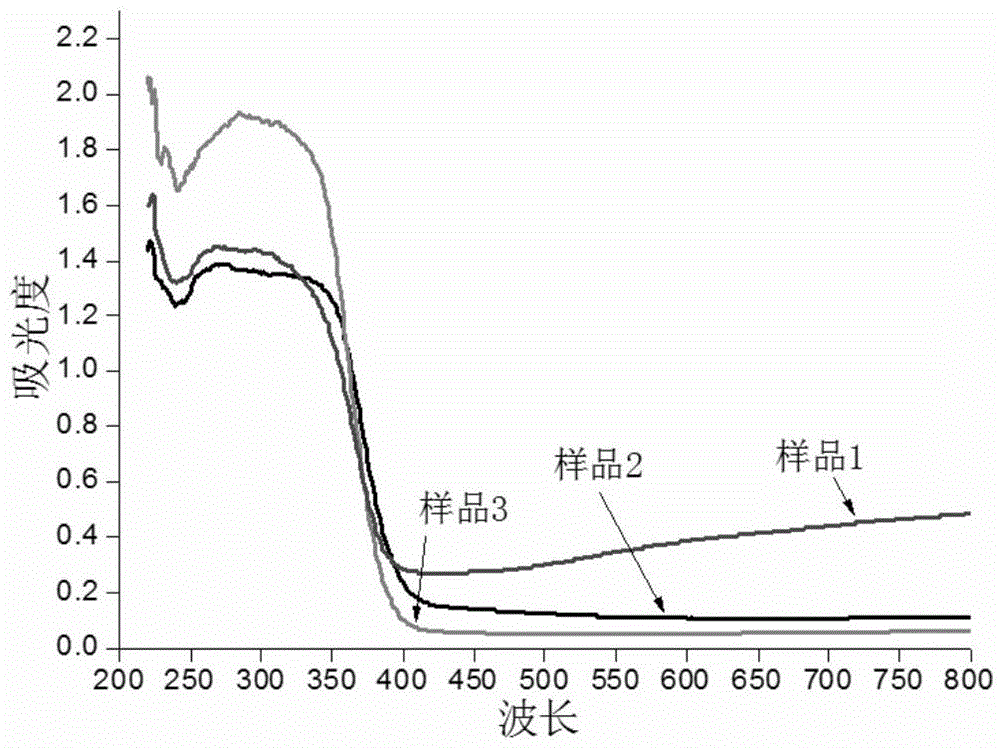
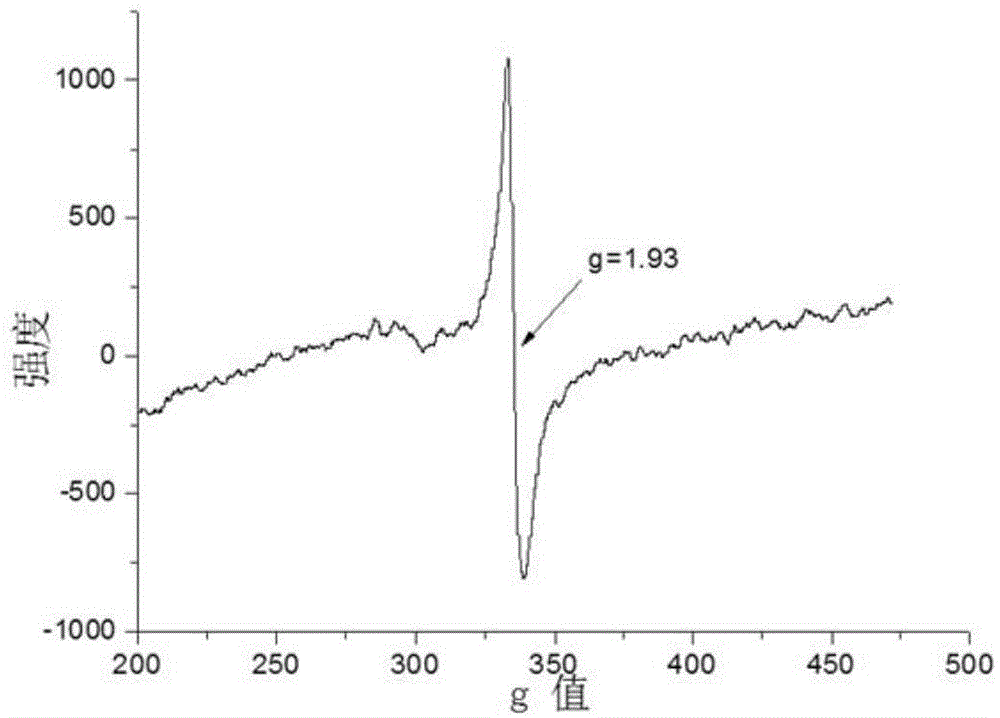
Method for preparing blue titanium dioxide
A titanium dioxide and blue technology is applied in the field of inorganic nano-photocatalyst materials to achieve the effects of simple method, high yield and good optical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The present embodiment blue titanium dioxide is prepared according to the following steps:
[0044] A, in ice bath, 2.32 grams of anhydrous titanium tetrachloride are dissolved in 5ml absolute ethanol, and the obtained mass concentration is 37% titanium tetrachloride ethanolic solution;
[0045]b. At room temperature, add 2ml of titanium trichloride to 5ml of absolute ethanol to obtain mixed solution A, then add 0.3g of sodium fluoride powder to mixed solution A to obtain mixed solution B, titanium trichloride and fluoride The molar ratio of sodium powder is 2:1;
[0046] C, under room temperature, add the titanium tetrachloride ethanol solution that 0.18ml step a gains in the mixed solution B gained in step b, obtain mixed solution C, wherein the mol ratio of titanium tetrachloride and titanium trichloride is 1: 40;
[0047] d. Put the mixed liquid C into a hydrothermal reaction kettle and place it in an oven, react at a temperature of 180°C for 12 hours to obtain a ...
Embodiment 2
[0057] The present embodiment blue titanium dioxide is prepared according to the following steps:
[0058] A, in ice bath, 3 grams of anhydrous titanium tetrachloride are dissolved in 8ml dehydrated alcohol, the obtained mass concentration is the titanium tetrachloride ethanol solution of 32.2%;
[0059] b. At room temperature, add 10ml of titanium trichloride to 25ml of absolute ethanol to obtain mixed solution A, then add 1.5g of sodium fluoride powder to mixed solution A to obtain mixed solution B, titanium trichloride and fluoride The molar ratio of sodium powder is 2:1;
[0060] C, under room temperature, add the titanium tetrachloride ethanol solution that 0.89ml step a gains in the mixed solution B gained in step b, obtain mixed solution C, wherein the mol ratio of titanium tetrachloride and titanium trichloride is 1: 50;
[0061] d. Put the mixed liquid C into a hydrothermal reaction kettle and put it in an oven, react at a temperature of 180°C for 15 hours to obtain...
Embodiment 3
[0065] The present embodiment blue titanium dioxide is prepared according to the following steps:
[0066] A, in ice bath, 4 grams of anhydrous titanium tetrachloride are dissolved in 10ml absolute ethanol, and the obtained mass concentration is 33.6% titanium tetrachloride ethanolic solution;
[0067] b. At room temperature, add 37.5ml of titanium trichloride to 90ml of absolute ethanol to obtain mixed solution A, then add 6.5g of sodium fluoride powder to mixed solution A to obtain mixed solution B, titanium trichloride and fluorine The molar ratio of sodium chloride powder is 2:1;
[0068] c, at room temperature, add 2.5ml of the titanium tetrachloride ethanol solution obtained in step a to the mixed solution B obtained in step b, to obtain mixed solution C, wherein the mol ratio of titanium tetrachloride and titanium trichloride is 1: 60;
[0069] d. Put the mixed liquid C into a hydrothermal reaction kettle and put it in an oven, react at a temperature of 180°C for 15 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com