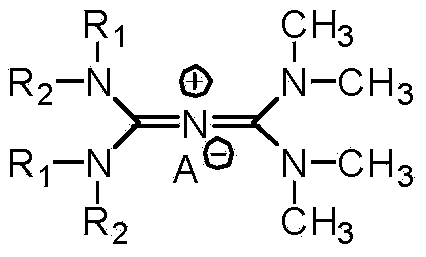
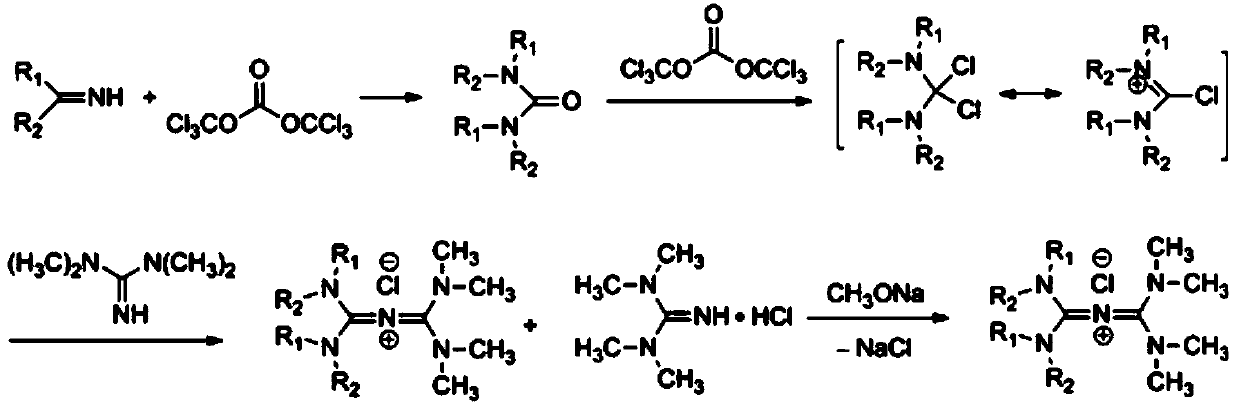
Preparation method of N-alkyl conjugated ion type quaternary ammonium salt
A technology of conjugated ions and quaternary ammonium salts, which is applied in the field of preparation of quaternary ammonium salt phase transfer catalysts, can solve the problems of large amount of reaction solvent used, reaction yield greatly affected by ambient humidity, and lengthy catalyst synthesis process steps. Achieve the effect of reducing production cost, shortening reaction time, and increasing reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Carbonylation reaction: In a flask equipped with a stirrer, a reflux condenser, and a constant pressure dropping funnel, add 225g of an aqueous solution of dimethylamine (40%), and dropwise add 118.7g of bis(trichlorohydrin) under vigorous stirring at 10°C. Toluene solution of methyl)carbonate, and adding 220g of acid-binding agent triethylamine, after the dropwise addition, rise to room temperature and stir for 4h, separate the organic phase, extract the water phase with toluene, combine the organic phases, and distill under reduced pressure to obtain tetramethyl Base urea 95g, yield 80%, purity 98%.
[0035] Chlorination reaction: In a flask equipped with a stirrer, a reflux condenser, and a constant pressure dropping funnel, add 30 g of the above-mentioned tetramethylurea, 30 g of toluene, and add 30 g of bis(trichloromethyl)carbonate dropwise at 5°C. The toluene solution was added dropwise for 4h, raised to room temperature and stirred for 2h. Filter and wash the f...
Embodiment 2
[0038] In a flask equipped with a stirrer, a reflux condenser, and a constant pressure dropping funnel, add 146g of diethylamine, slowly add dropwise a toluene solution containing 120g of bis(trichloromethyl)carbonate under vigorous stirring at 5°C, and Add 185 g of acid-binding agent pyridine. After the dropwise addition, raise to room temperature and stir for 4 h, and distill under reduced pressure to obtain tetraethylurea. Yield 91%, purity 98%.
[0039] In a flask equipped with a stirrer, a reflux condenser, and a constant pressure dropping funnel, add 43 g of the above-mentioned tetraethylurea, 40 g of toluene, and dropwise add a toluene solution containing 30 g of bis(trichloromethyl)carbonate at 5° C. Add dropwise for 4h, rise to room temperature and stir for 2h. After filtering, the filter cake was washed with toluene to obtain 41 g of chlorinated salt.
[0040] Dissolve the chlorinated salt in 60g of dichloromethane, add 50g of tetramethylguanidine dropwise at 10°C,...
Embodiment 3
[0042] In a flask equipped with a stirrer, a reflux condenser, and a constant pressure dropping funnel, add 130 g of dibutylamine, and slowly add a toluene solution containing 60 g of bis(trichloromethyl)carbonate dropwise under vigorous stirring at 5°C, and Add 125 g of acid-binding agent triethylamine, after the dropwise addition, raise to room temperature and stir for 4 h, then distill under reduced pressure to obtain tetrabutylurea. Yield 90%, purity 98%.
[0043] In a flask equipped with a stirrer, a reflux condenser, and a constant pressure dropping funnel, add 73 g of the above-mentioned tetrabutylurea, 40 g of toluene, and add dropwise a toluene solution containing 30 g of bis(trichloromethyl)carbonate at 5° C. Add dropwise for 4h, rise to room temperature and stir for 2h. After filtering, the filter cake was washed with toluene to obtain 70 g of chlorinated salt.
[0044] Dissolve the chlorinated salt in 60g of dichloromethane, add 50g of tetramethylguanidine dropwi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com