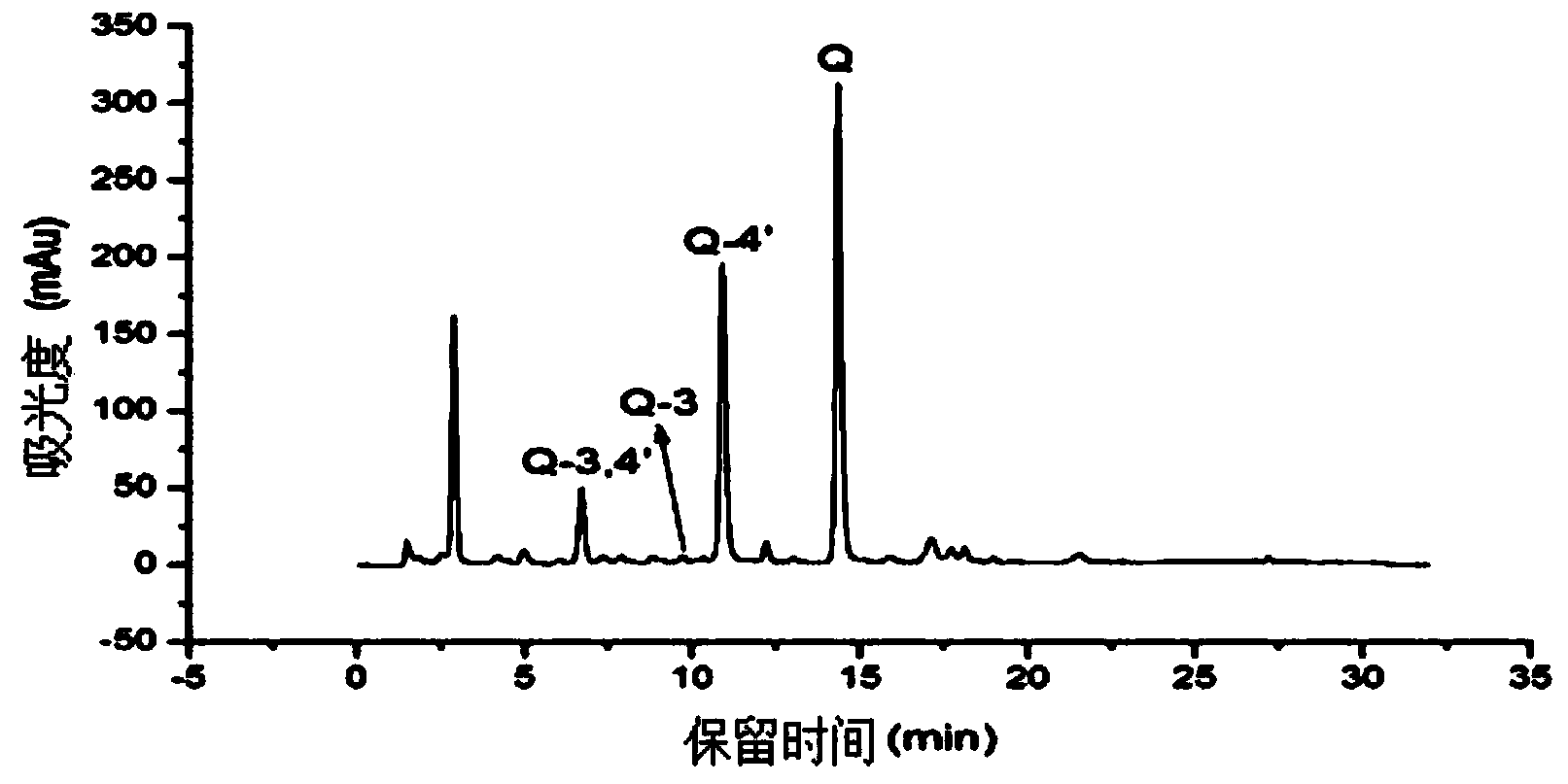
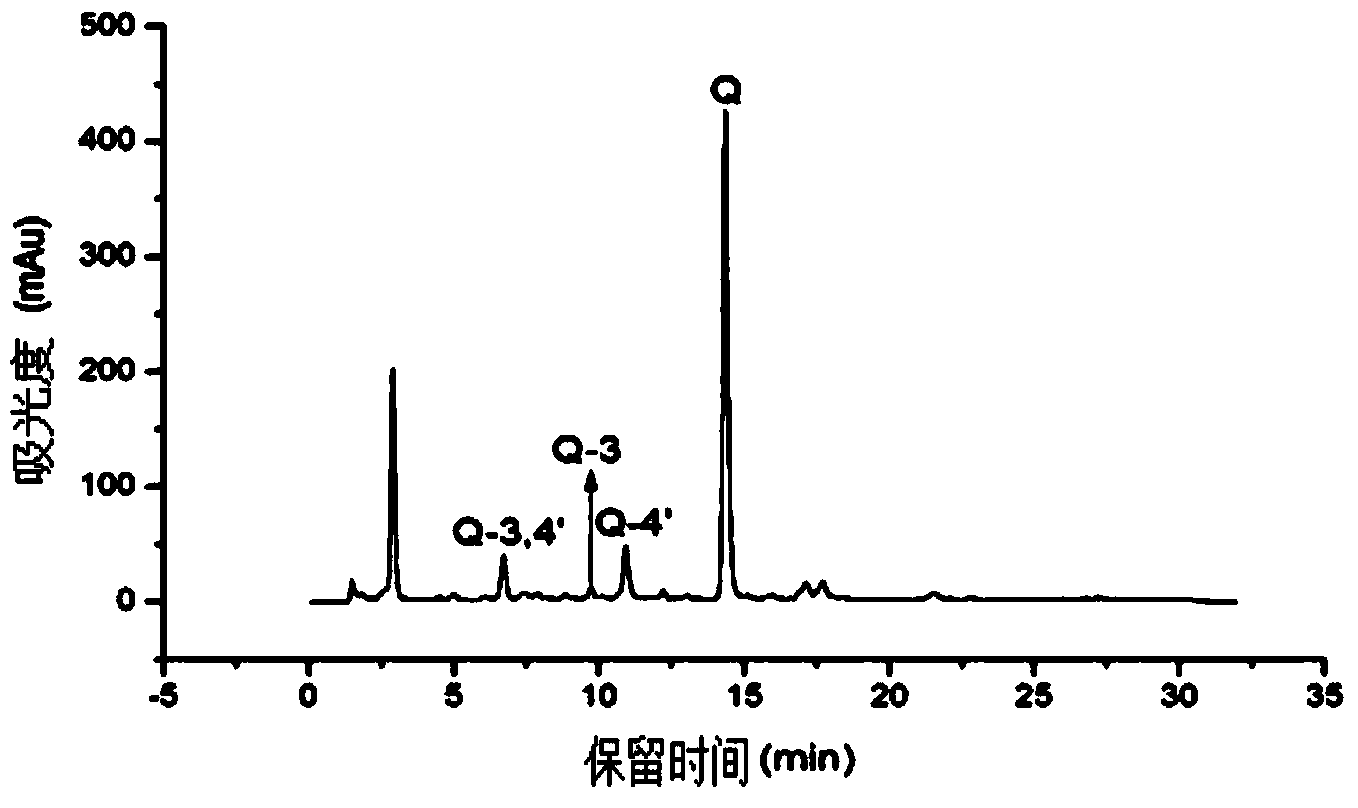
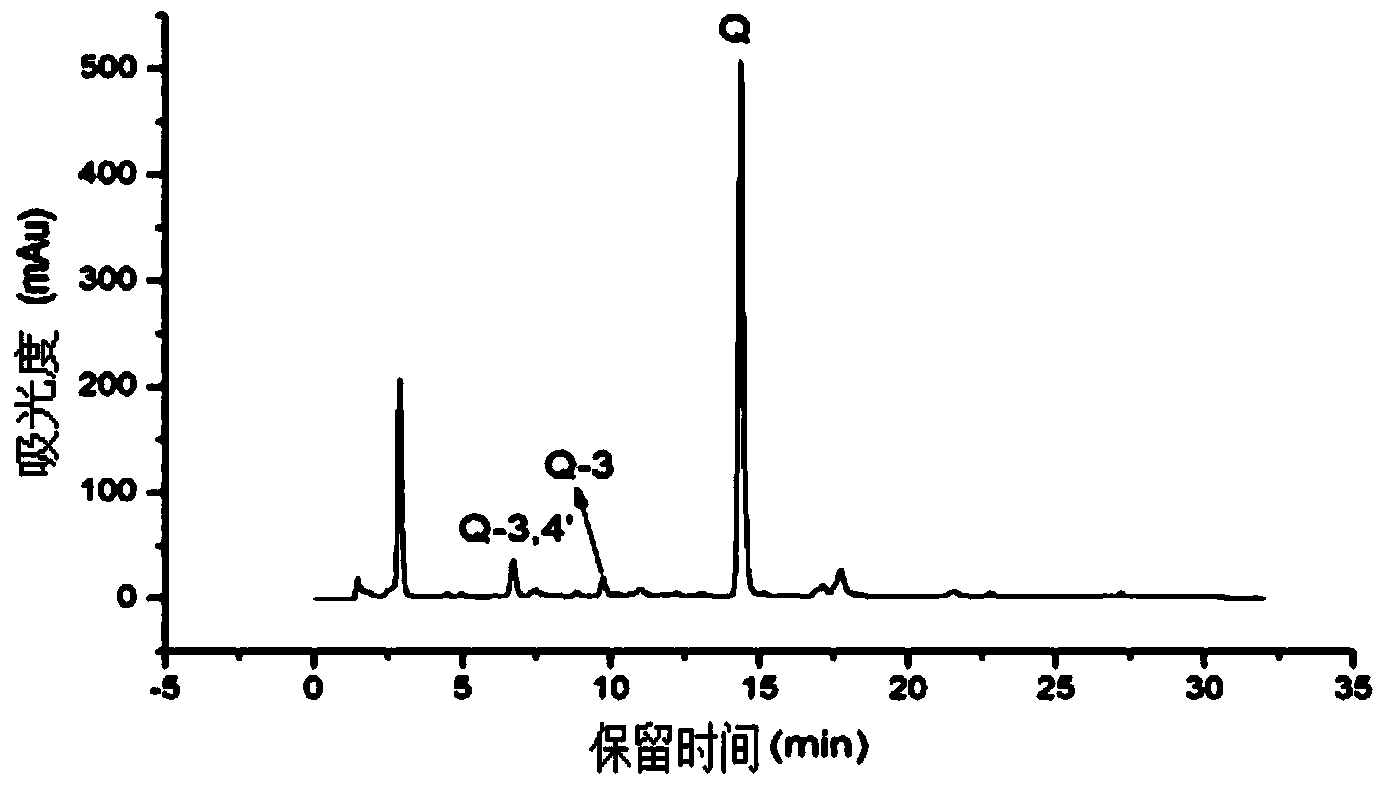
Application of heat-resistant beta-glucosidase and mutants thereof
A technology of glucosidase and heat resistance, applied in the direction of glycosylase, enzyme, hydrolase, etc., can solve the problem of low specificity, achieve high conversion efficiency, less reaction by-products, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] According to the β-glucosidase gene sequence of family 1 of Thermotoga (Thermotiga), sequence number: X74163), design mutation primers, specifically
[0026] 5'-AGTTTTCAACAACACCTATTTCGAACCTGCGAGT-3' (SEQ ID NO. 4) and 5'-ATTCCGATCTTTCCATCTTTCACG-3' (SEQ ID NO. 5);
[0027]The recombinant plasmid pET-20b-bglA (J Ind Microbiol Biotechnol, 2009, 36:1401–1408) containing the wild-type β-glucosidase gene of Thermotoga was selected as a template for inverse PCR amplification to obtain β-glucosidase The nucleotide fragment of the enzyme mutant gene is phosphorylated, connected, transformed, and the plasmid is extracted to obtain a recombinant plasmid containing the G224T nucleotide fragment, and the host is further transformed to express it, and the β-glucoside of the present invention is obtained The amino acid sequence of the enzyme mutant G224T is shown in SEQ ID NO.1.
Embodiment 2
[0029] It is basically the same as Example 1, except that the primers used are: 5'-AGTTTTCAATAGTGGATATTTCGAACCTGCGAGT-3' (SEQ ID NO.6) and 5'-ATTCCGATCTTTCCATCTTTCACG-3' (SEQ ID NO.7), to obtain The recombinant plasmid of the nucleotide fragment is further transformed into a host to express it, thereby obtaining the β-glucosidase mutant N223S of the present invention; its amino acid sequence is shown in SEQ ID NO.2.
Embodiment 3
[0031] It is basically the same as Example 1, except that the primers used are: 5'-AGTTTTCAATAGTACCTATTTCGAACCTGCGAGT-3' (SEQ ID NO.8) and 5'-ATTCCGATCTTTCCATCTTTCACG-3' (SEQ ID NO.9), to obtain The recombinant plasmid of / G224T nucleotide fragment is further transformed into a host to express it, thereby obtaining the β-glucosidase mutant N223S / G224T of the present invention; its amino acid sequence is shown in SEQ ID NO.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com