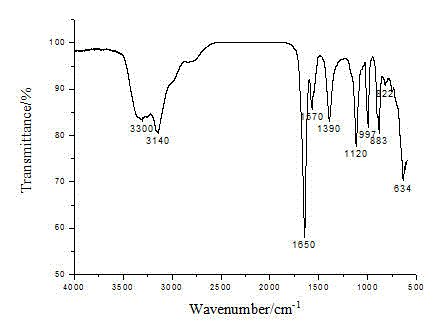
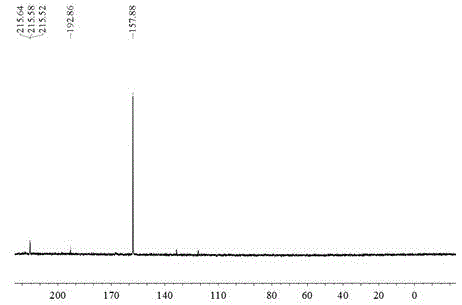
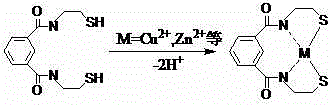Heavy metal chelating trapping sulfide agent hexasulfoguanidinoformic acid or salt thereof, and preparation method and application thereof
A technology of hexathioguanidine formic acid and hexathioguanidine, which is applied in the field of sulfide heavy metal chelating agent hexathioguanidine formic acid or its salts, can solve the problem of reducing the removal effect of heavy metal ions and sulfide precipitation Small particles, long sedimentation time and other problems, to achieve the effect of not easy secondary pollution, large sedimentation particles, and good treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Add 2L of water, 790g of potassium hydroxide, and 100g of solid potassium phosphate into the reactor, stir to dissolve, then add 287g of guanidine hydrochloride, the ratio of alkali to guanidine hydrochloride is 4:1, and stir for 15 minutes below 10°C . Then slowly add 540mL carbon disulfide and 500mL acetone mixed solution dropwise, the ratio of the amount of carbon disulfide to guanidine hydrochloride is 3:1, the drop rate is controlled during the dropwise addition, so that the reaction temperature is maintained below 10°C. CS 2 After the dropwise addition was completed, the temperature was raised to 40° C. and the reaction was stirred at a constant temperature for 2 hours. The obtained brown-red liquid was cooled, crystallized and filtered, and the filtrate was extracted with acetone and ethanol respectively, and the final product was dried to constant weight to obtain 792 grams of potassium hexathioguanidinoformate, with a reaction yield of 65.8%.
Embodiment 2
[0063] Add 2L of water, 790g of potassium hydroxide, and 100g of solid potassium phosphate into the reactor, stir to dissolve, then add 287g of guanidine hydrochloride, the ratio of alkali to guanidine hydrochloride is 4:1, and stir for 15 minutes below 10°C . Then slowly add 630mL of carbon disulfide and 500mL of acetone mixed solution dropwise, the ratio of the amount of carbon disulfide to guanidine hydrochloride is 3.5:1, and the drop rate is controlled during the dropwise addition to keep the reaction temperature below 10°C. CS 2 After the dropwise addition was completed, the temperature was raised to 40° C. and the reaction was stirred at a constant temperature for 2 hours. Obtain a brownish-red liquid, cool, crystallize and filter, add 200mL concentrated hydrochloric acid to the filtrate and extract with acetone and ethanol respectively, and finally the product is dried to constant weight to obtain 592 grams of hexathioguanidine formic acid, the reaction yield is 70% ...
Embodiment 3
[0065] Add 2L of water, 790g of potassium hydroxide, and 100g of solid potassium phosphate into the reactor, stir to dissolve, then add 287g of guanidine hydrochloride, the ratio of alkali to guanidine hydrochloride is 4:1, and stir for 15 minutes below 10°C . Then slowly add 720mL of carbon disulfide and 500mL of acetone mixed solution dropwise, the ratio of the amount of carbon disulfide to guanidine hydrochloride is 4:1, and the drop rate is controlled during the dropwise addition to keep the reaction temperature below 10°C. CS 2 After the dropwise addition was completed, the temperature was raised to 40° C. and the reaction was stirred at a constant temperature for 3 hours. The brownish-red liquid was obtained, crystallized by cooling and filtered, and the filtrate was extracted with acetone and ethanol respectively, and the final product was dried to constant weight to obtain 892 grams of potassium hexathioguanidinoformate, with a reaction yield of 74.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com