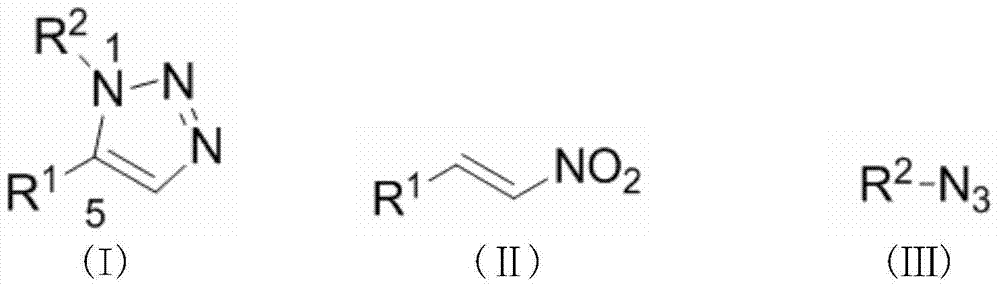
Synthesis method for substituted triazole compound
A compound and catalyst technology, applied in the field of synthesis of triazole compounds, can solve the problems of limited application of catalysts, slow cycloaddition reaction, etc., and achieve the effects of simple operation and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
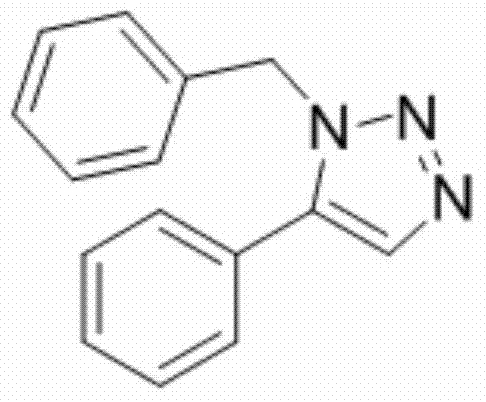
[0020] Example 1: Synthesis of 1-benzyl-5-phenyl-1H-1,2,3-triazole
[0021] Add 1 mmol of β-nitrostyrene, 1 mmol of benzyl azide, 0.05 mmol of cerium trifluoromethanesulfonate and 2 mL of toluene into a 25 mL round bottom flask, and reflux the reaction at 110 ° C until complete (TLC tracking detection, about 10 h ), stop the reaction, wait for the reaction solution to be cooled to room temperature, filter, remove the solvent under reduced pressure, and purify the residue by fast silica gel column chromatography (eluent is ethyl acetate / petroleum ether=1:20, volume ratio), to obtain light Yellow oily liquid 1a, yield 85%.
[0022] Gained pale yellow oily liquid 1a is analyzed, and its spectral characteristics are as follows:
[0023] 1 H NMR (400MHz, CDCl 3 ): δ7.74(s,1H),7.47-7.34(m,3H),7.27(m,5H),7.11-7.03(m,2H),5.55(s,2H)ppm;
[0024] 13 C NMR (100MHz, CDCl 3 )δ138.15, 135.49, 133.27, 129.50, 128.94, 128.88, 128.81, 128.14, 127.14, 126.89, 51.80ppm;
[0025] HRMS(m / z)...
Embodiment 2
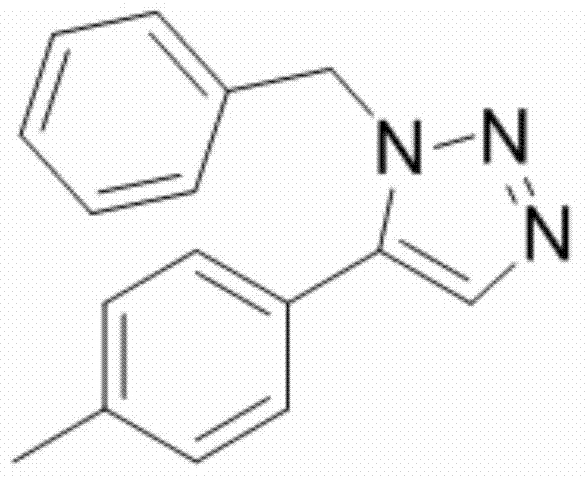
[0028] Example 2: Synthesis of 1-benzyl-5-(4-methylphenyl)-1H-1,2,3-triazole
[0029] Add 1mmol of 4-methyl-β-nitrostyrene, 1mmol of benzyl azide, 0.08mmol of samarium trifluoromethanesulfonate and 5mL of toluene into a 25mL round bottom flask, and reflux the reaction at 100°C until complete (TLC tracking detection , about 12h), stop the reaction, wait for the reaction solution to cool to room temperature, filter, remove the solvent under reduced pressure, and purify the residue by flash silica gel column chromatography (eluent is ethyl acetate / petroleum ether=1:10, volume ratio) , a yellow oily liquid 1b was obtained with a yield of 80%.
[0030] Gained yellow oily liquid 1b is analyzed, and its spectral characteristics are as follows:
[0031] 1 H NMR (400MHz, CDCl 3 ):δ7.71(s,1H),7.29(m,3H),7.22(d,J=8.0Hz,2H),7.14(d,J=8.1Hz,2H),7.12-7.02(m,2H) ,5.53(s,2H),2.39(s,3H)ppm;
[0032] 13 C NMR (100MHz, CDCl 3 ): δ139.62, 138.22, 135.65, 133.11, 129.63, 128.79, 128.75, 128....
Embodiment 3
[0036] Example 3: Synthesis of 1-benzyl-5-[4-(benzyloxy)-3-methoxyphenyl]-1H-1,2,3-triazole
[0037] Add 1 mmol of trans-3-benzyloxy-4-methoxy-β-nitrostyrene, 1 mmol of benzyl azide, 0.01 mmol of europium trifluoromethanesulfonate, and 2 mL of toluene into a 25 mL round-bottomed flask at 80 °C Under the conditions, the reaction was refluxed until complete (TLC tracking detection, about 48h), the reaction was stopped, and the reaction solution was cooled to room temperature, filtered, and the solvent was removed under reduced pressure, and the residue was purified by fast silica gel column chromatography (eluent was ethyl acetate / Petroleum ether=1:25, volume ratio), to obtain yellow oily liquid 1c, yield 71%.
[0038] Gained yellow oily liquid 1c is analyzed, and its spectral characteristics are as follows:
[0039] yellow oil; 1 H NMR (400MHz, CDCl 3 ):δ7.69(s,1H),7.53-7.18(m,8H),7.16-7.01(m,2H),6.90(d,J=8.3Hz,1H),6.76(dd,J=8.2,2.0 Hz,1H),6.62(d,J=1.9Hz,1H),5.52(s,2H),5.18(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com