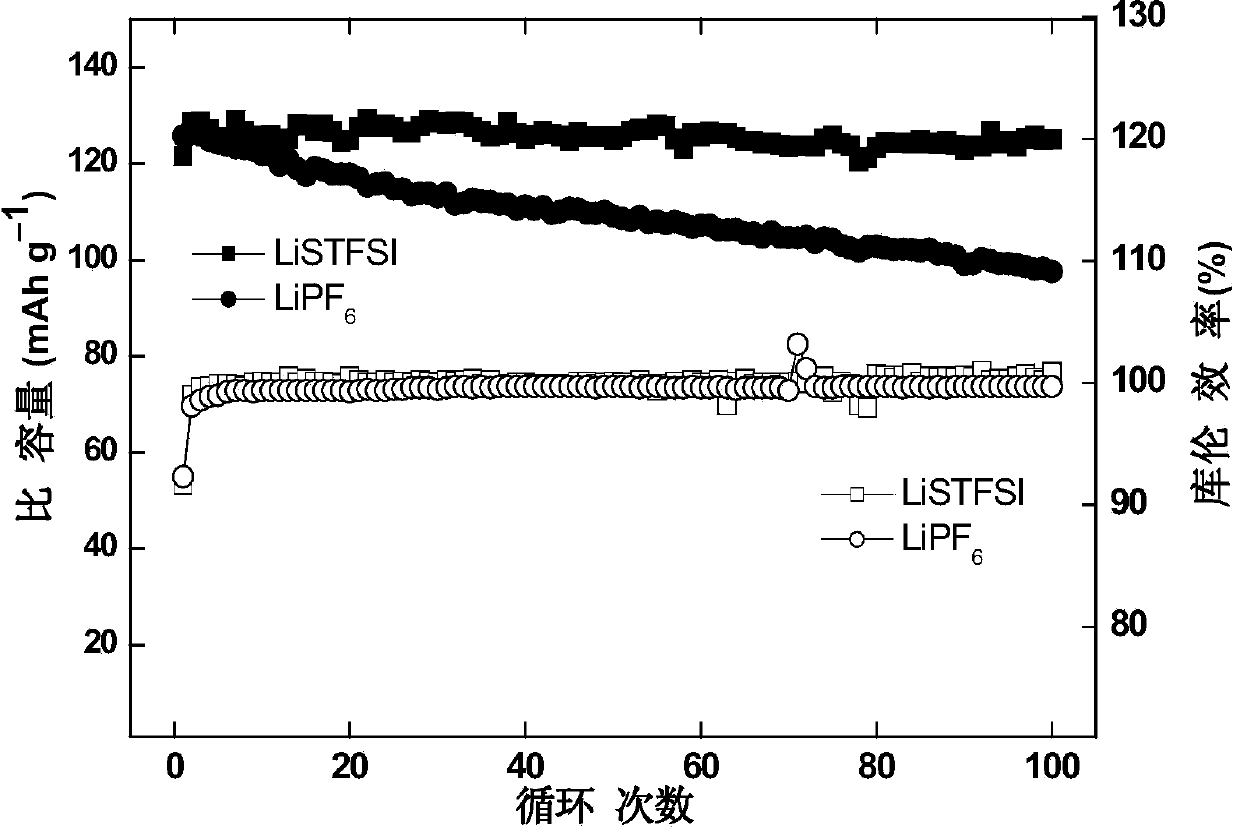
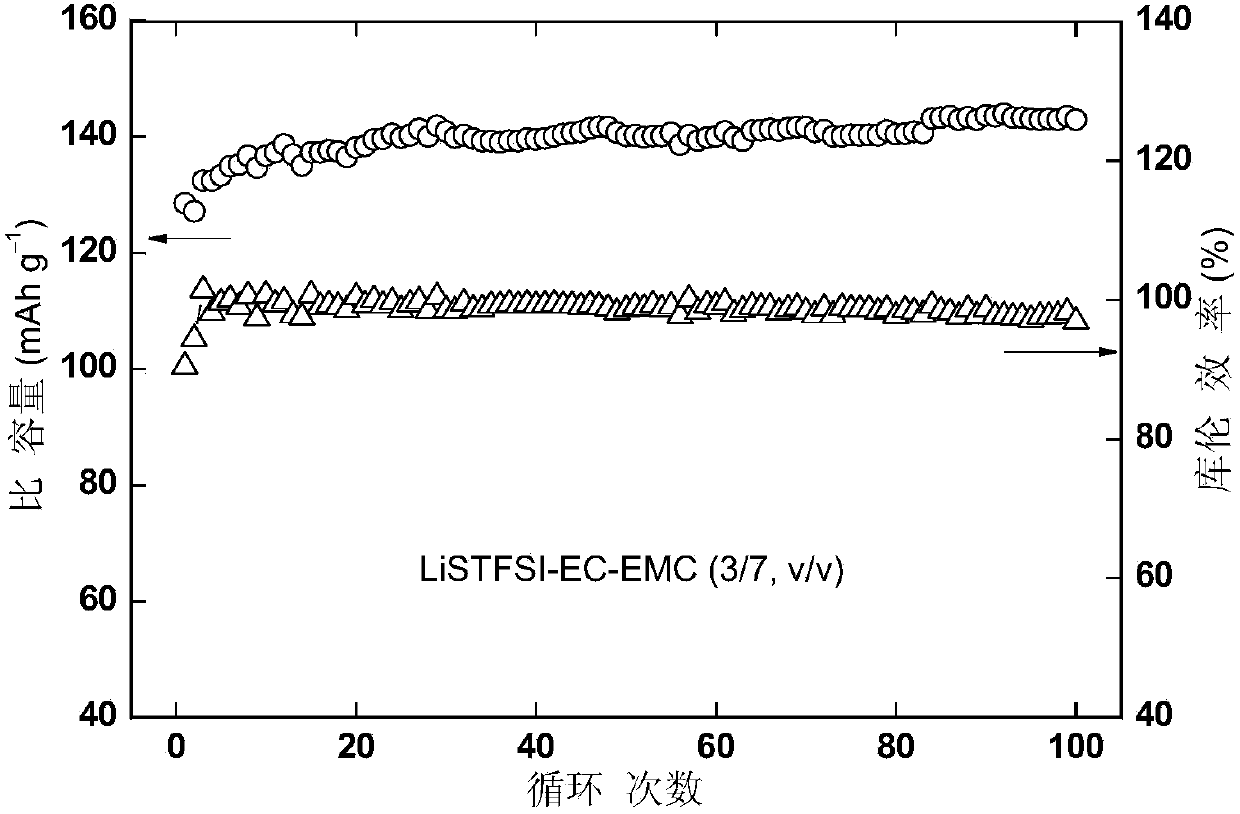
Imine alkali metal salt and ion liquid as well as application thereof as non-water electrolyte
A technology of ionic liquids and electrolytes, applied in the preparation of sulfonamides, organic chemistry, etc., can solve the problems of long synthetic routes, low overall yields, and high cost of preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0115] Embodiment 1-9 relates to the preparation of imide alkali metal salt containing "S-fluoroalkylsulfonylimide group"
Embodiment 1
[0116] Embodiment 1: (trifluoromethylsulfonyl) (trifluoromethylsulfinyl) potassium imide ([(CF 3 SO 2 )(SOCF 3 )N]K) The preparation synthesis reaction route is as follows:
[0117]
[0118] Add sodium trifluoromethanesulfinate (CF 3 SO 2 Na) (65.5g, 0.42mol), 100mL chlorobenzene. Stir mechanically, heat to 30°C, slowly add SOCl dropwise 2 (59.5 g, 0.5 mol), stirring was continued for 2 hours after the dropwise addition was completed. Atmospheric distillation, collecting fractions at 30°C. 55 g of trifluoromethanesulfonyl chloride (pale brown liquid) were obtained, yield 87%.
[0119] Add trifluoromethanesulfonamide (CF 3 SO 2 NH 2 ) (29.8g, 0.2mol), acetonitrile (80mL), pyridine (31.6g, 0.4mol) as acid-binding agent, stirred to dissolve, and added dropwise trifluoromethylsulfinyl chloride (CF 3 SOCl) (35g, 0.23mol), remove the ice bath after the dropwise addition, continue to stir at room temperature for 24h, and remove the solvent and unreacted CF by rotary eva...
Embodiment 2
[0120] Example 2: Trifluoromethyl (S-trifluoromethylsulfonylimido) sulfonamide (CF 3 (CF 3 SO 2 N)SONH 2 ) preparation
[0121]
[0122] Potassium (trifluoromethylsulfonyl)(trifluoromethylsulfinyl)imide ([(CF 3 SO 2 )(SOCF 3 )N]K) (66.7g, 0.22mol) was dissolved in 200mL distilled water, and was added successively under mechanical stirring 2 OSO 3 H) (38.2g, 0.34mol) and sodium acetate (CH 3 CO 2 Na) (27.9g, 0.34mol), after stirring for 8 hours, extracted with ether (50mLx3), and combined the organic phases. After adding anhydrous magnesium sulfate to dry, filter, collect the filtrate, and remove ether by rotary evaporation to obtain a white flaky solid. Yield 34.8g, yield 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com