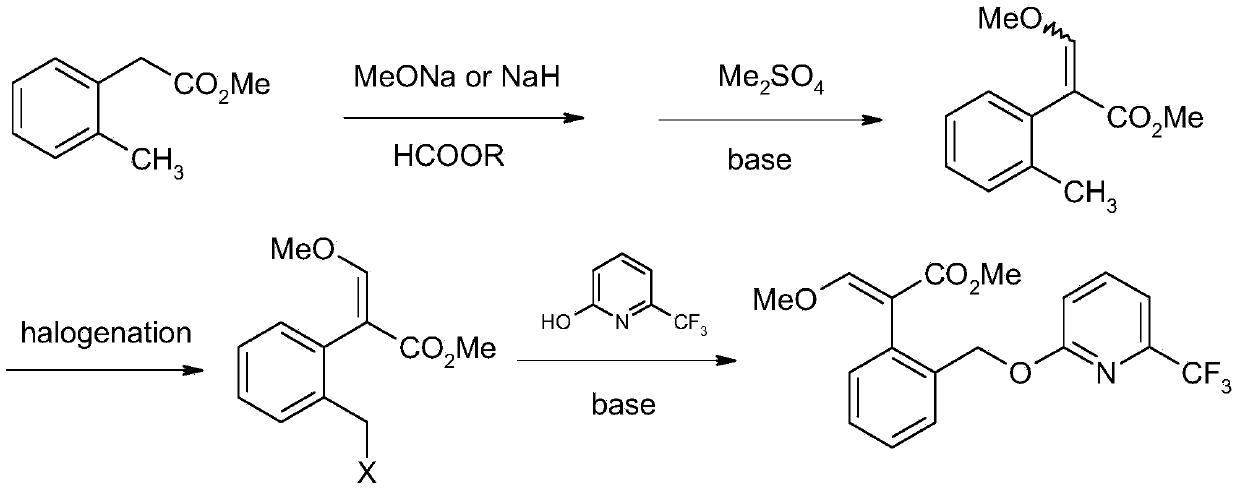
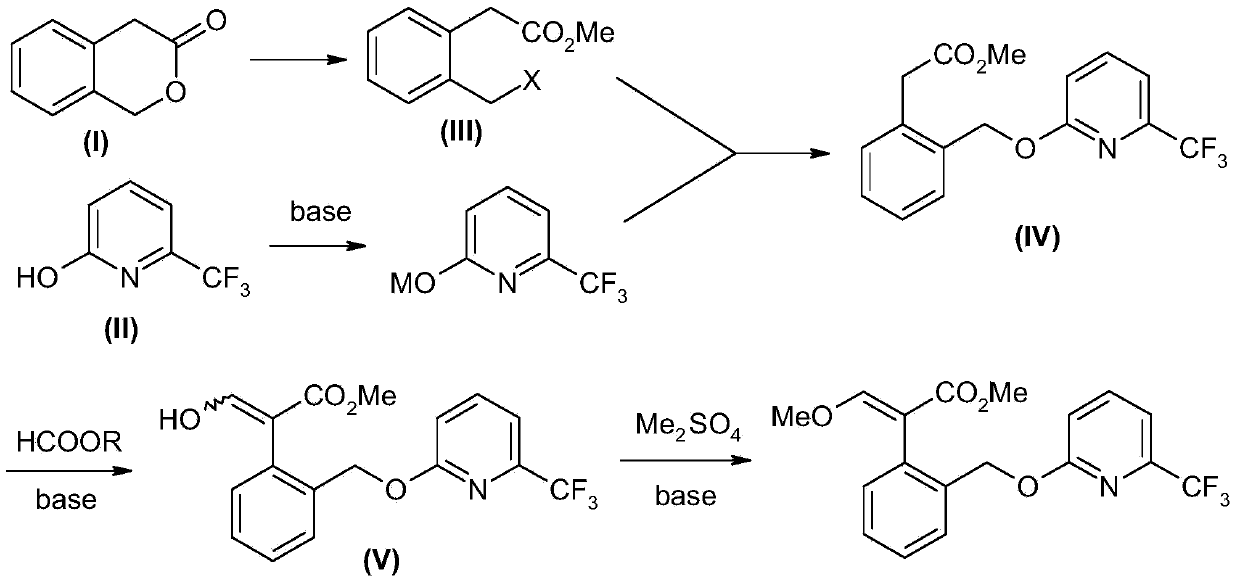
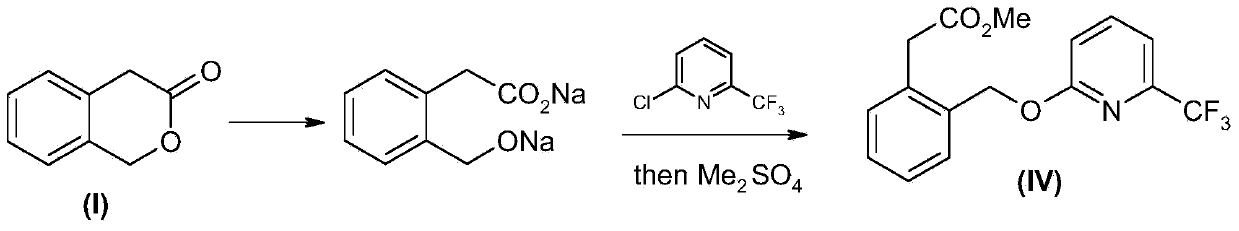
Picoxystrobin preparation method
A technology of picoxystrobin and oxygen, which is applied in the field of preparation of the fungicide picoxystrobin, can solve the problem of low reaction yield, low yield of picoxystrobin phenyl acetate, and influence on the final product yield and other problems, to achieve the effect of mild reaction conditions, good selectivity, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1), 44.4 grams (0.3mol) of compound (I) 3-isochromone was dissolved in a mixture of 45 ml of methanol and 150 ml of toluene, cooled to below 5°C, and 46 ml (0.63mol) was added dropwise under control of 10°C Thionyl chloride, the tail gas was absorbed with 30% hydrogen peroxide, and after the addition was completed, the temperature was naturally raised to room temperature and continued to stir for 2 hours. Control the temperature below 30°C, depressurize the water pump, add hydrogen peroxide absorption liquid into the safety bottle connected to the water pump, and remove low boiling substances. The remaining toluene liquid was washed successively with 25 ml of water and 25 ml of saturated aqueous sodium bicarbonate solution, and the toluene was concentrated and recovered to obtain 62.7 g of crude product of compound (III) (2′-halomethyl)phenylacetate methyl ester, which can be directly used in the next step reaction.
[0051]
[0052] 2) Suspend 48.9 g (0.3 mol) of c...
Embodiment 2
[0058] Embodiment 2 (comparative experiment with embodiment 1 step 2)
[0059] 1.63 g (0.01 mol) of compound (II) 6-trifluoromethyl-2-hydroxypyridine was dissolved in 10 ml of DMF, 1.5 g (0.011 mol) of anhydrous potassium carbonate was added, and stirred at room temperature for 1 hour. 0.01 mol of compound (III) was added dropwise, and the temperature was controlled below 30°C. After the addition, continue stirring at room temperature for 2 hours, filter, concentrate under reduced pressure to recover DMF, add 20 ml of toluene to the residue, wash with 5 ml of 5% dilute hydrochloric acid and 5 ml of saturated sodium bicarbonate successively, concentrate and recover toluene, column chromatography (acetic acid Ethyl ester:petroleum ether=1:5) to obtain 2.54 g of compound (IV) with a yield of 78.1%. 1 HNMR (CDCl 3 ), δ3.67 (s, 3H), 3.84 (s, 2H), 5.46 (s, 2H), 6.89 (d, 1H), 7.23-7.31 (m, 4H), 7.51-7.53 (m, 1H), 7.66-7.70 (m, 1H).
Embodiment 3
[0060] Embodiment 3 (comparative experiment with embodiment 1 step 2)
[0061] 1.63 g (0.01 mol) of compound (II) 6-trifluoromethyl-2-hydroxypyridine was dissolved in 10 ml of DMF, 1.17 g (0.011 mol) of anhydrous sodium carbonate was added, and stirred at room temperature for 2 hours. 0.01 mol of compound (III) was added dropwise, and the temperature was controlled below 30°C. After the addition, continue to stir at room temperature for 8 hours, filter, concentrate under reduced pressure to recover DMF, add 20 milliliters of toluene to the residue, wash with 5 milliliters of 5% dilute hydrochloric acid and 5 milliliters of saturated sodium bicarbonate successively, concentrate and recover toluene, column chromatography (acetic acid Ethyl ester:petroleum ether=1:5) to obtain 1.37 g of compound (IV) with a yield of 42.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com