Copolymer containing thiophene-benzobis(benzothiadiazole), preparation method thereof and applications thereof
A technology of benzothiadiazole and copolymer, which is applied in the field of thiophene-benzodiazole-containing copolymer and its preparation, can solve problems such as limiting the scope of application, and achieve expansion of absorption range, improvement of stability, improvement of solubility and The effect of molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
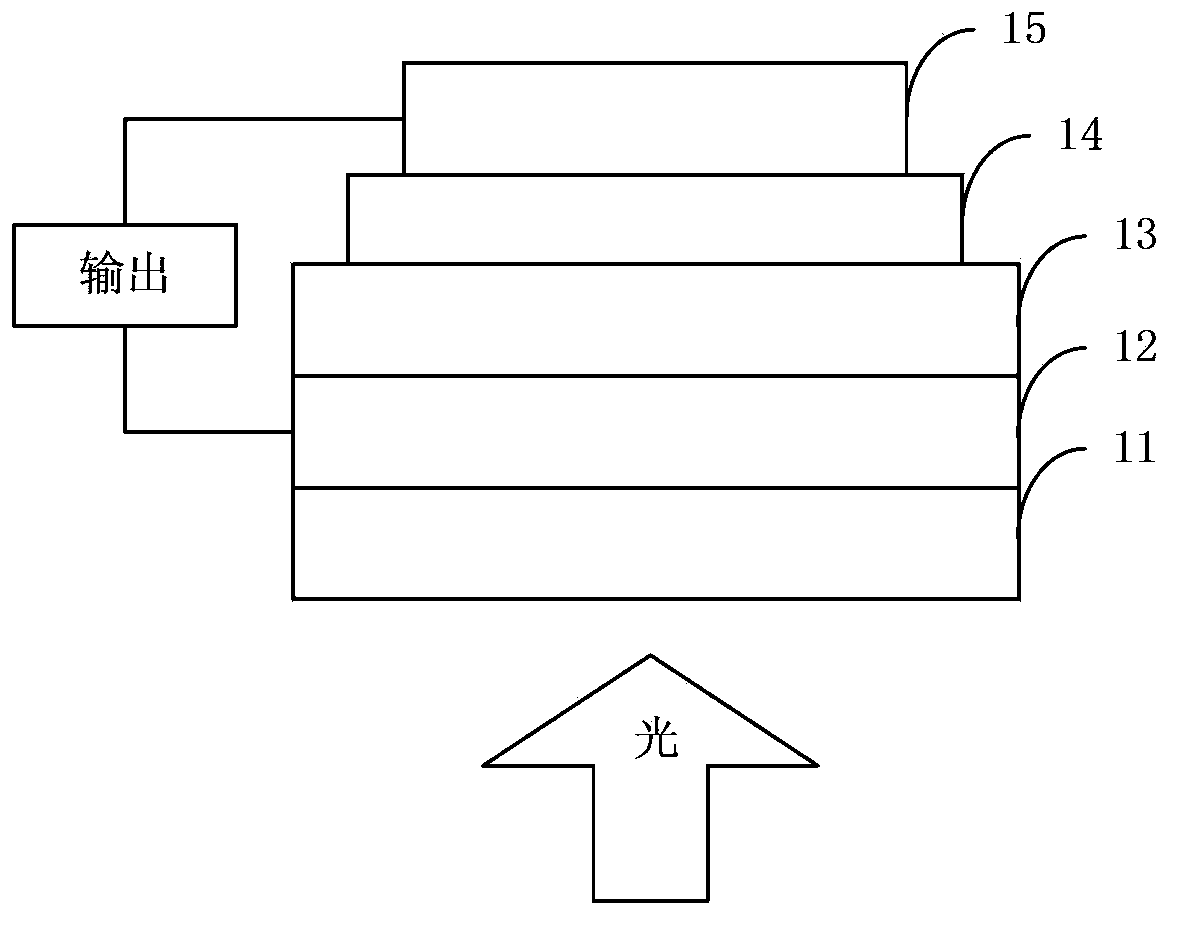
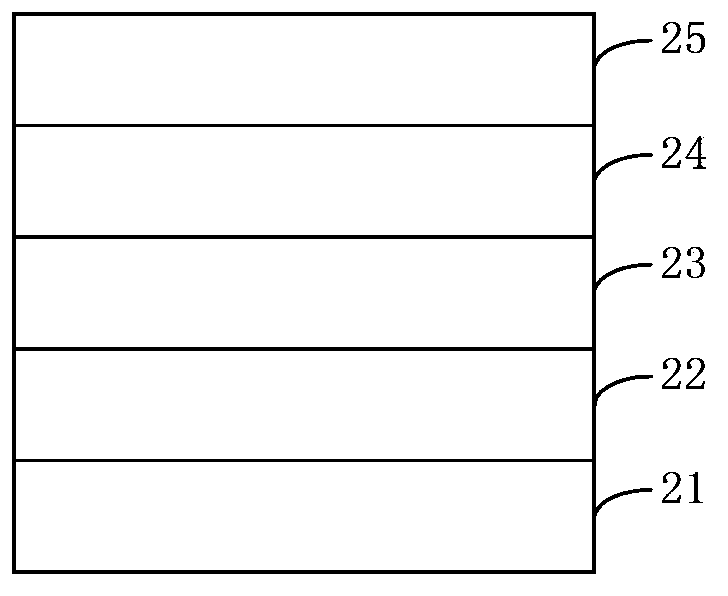
Image
Examples
preparation example Construction
[0035] The present invention also provides the preparation method of the above-mentioned copolymer containing thiophene-benzobis(benzothiadiazole), comprising the steps of:
[0036] S1, the preparation structural formula is Compound A (i.e. 5-nitro-2,1,3-benzothiadiazole):
[0037] 1. The structural formula is Compound E (4-nitrobenzene-1,2-diamine) was added to thionyl chloride, stirred and added to thionyl chloride (the structural formula is ) was added dropwise pyridine (the structural formula is Molecular formula is C 5 h 5 N,), reflux reaction at 85 ° C, stop the reaction, the obtained structural formula is Compound F (that is, 5-nitro-2,1,3 benzothiadiazole); the reaction formula is as follows:
[0038]
[0039] Compound F is preferably purified by:
[0040] Heating the reaction solution to 80°C and rotary evaporating excess SOCl 2 Afterwards, the reaction product was cooled to room temperature, poured into a large amount of water, stirred, filtered, washed...
Embodiment 1
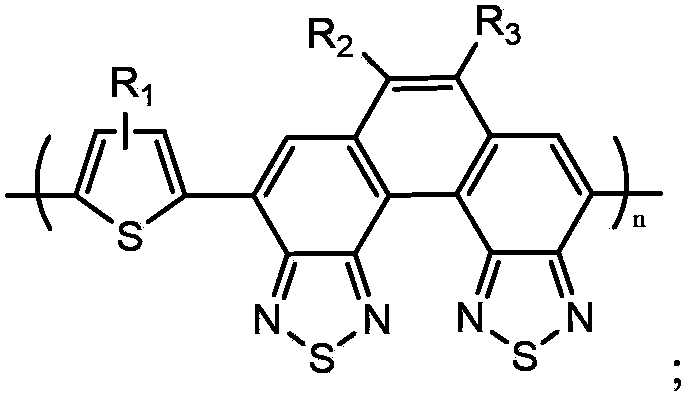
[0068] The copolymer containing thiophene-benzobis(benzothiadiazole) in this embodiment, that is, poly{3-hexylthiophene-6,7-bis(3,7-dimethyl)octyl-benzo[2 ,1-e:3,4-e] two (benzothiadiazole)}, its structural formula is as follows:
[0069] n=52
[0070] The preparation process is as follows:
[0071] 1. Preparation of 4,4'-dibromo-6,6'-diiodo-bi-2,1,3-benzothiadiazole
[0072] 1. Preparation of 5-nitro-2,1,3 benzothiadiazole
[0073]
[0074] Add 4-nitrobenzene-1,2-diamine (22.95g, 0.15mol) and 100ml of thionyl chloride into a three-necked flask, stir and slowly add 2ml of pyridine dropwise, heat and reflux at 85°C for 24h, stop Reaction, heated to 80 °C and rotary evaporated excess SOCl 2 Finally, the reaction product was cooled to room temperature, poured into a large amount of water, stirred, filtered, washed with water, and then dried in vacuum to obtain 21.7 g of the purified product 5-nitro-2,1,3-benzothiadiazole with a yield of 80%;
[0075] 2. Preparation of 4...
Embodiment 2
[0095] The copolymer containing thiophene-benzobis(benzothiadiazole) in this embodiment, that is, poly{3-thiophene-6,7-bis(3,7-dimethyl)octyl-benzo[2, 1-e: 3,4-e]bis(benzothiadiazole)}, its structural formula is as follows:
[0096] n=80
[0097] The preparation process is as follows:
[0098] 1. Preparation of 4,4'-dibromo-6,6'-diiodo-bi-2,1,3-benzothiadiazole
[0099] This step one is the same as step one in embodiment 1.
[0100] Two, the preparation of 2,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)thiophene
[0101]
[0102] Under the protection of nitrogen, add 2,5-dibromothiophene (7.26g, 0.03mol) into the three-necked flask, add 200ml of tetrahydrofuran solvent, and slowly inject n-butyllithium (25.2 mL, 2.5M, 0.06mol), continue to stir the reaction for 2h, and inject 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dihexa Oxaborane (13 mL, 0.06 mol), stirred overnight at room temperature. Saturated aqueous sodium chloride (30ml) was added to terminate the reaction, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com