N<epsilon>-(1-methylcyclopropyl-2-acrylamide)-lysine translation system and application thereof
A methyl ring, enamide technology, applied in the field of biochemistry, can solve problems such as affecting the conformation of the protein itself
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: chemical synthesis
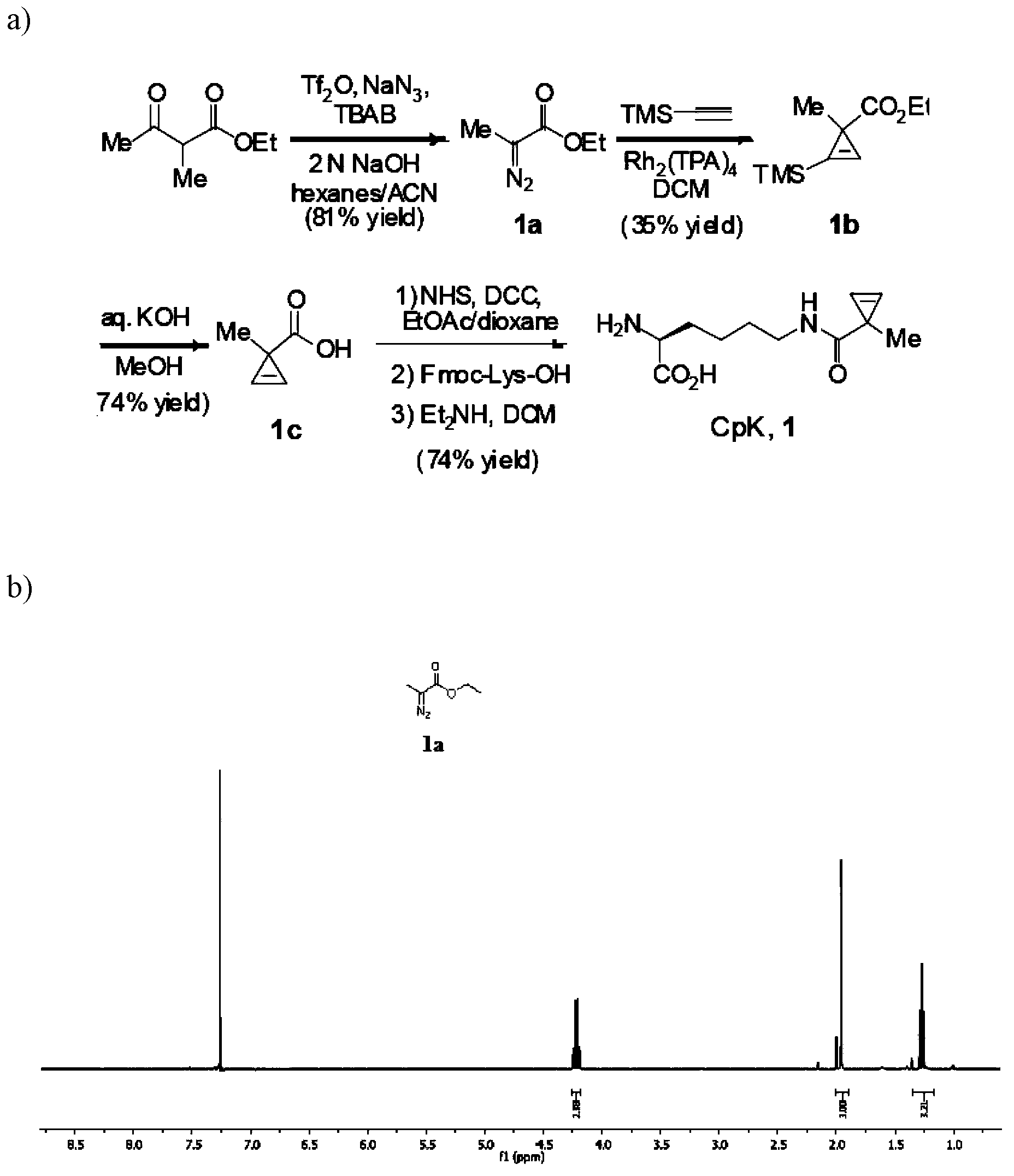
[0046] 1. N ε The chemical synthesis of -(1-methylcycloprop-2-enamide)-lysine (CpK)( figure 1 )
[0047] For the chemical synthesis route of CpK see figure 1 . Concrete synthesis reaction steps are as follows:
[0048] Into a 1000 mL round bottom flask equipped with a magnetic stirrer and a thermometer were added sodium azide (6.5 g, 100 mmol), 2M sodium hydroxide solution (200 mL, 40 mmol), tetrabutylammonium bromide (TBAB) (80 mg, 0.25 mmol ) and 100 mL of hexane (100 mL), stirred vigorously on an ice bath at 0°C. After complete dissolution, trifluoromethanesulfonic anhydride (8.2 mL, 50 mmol) was slowly added dropwise with a syringe, and reacted for 10 minutes. Another ethyl 2-methylacetoacetate (3.53mL, 25mmol) was dissolved in 100mL of acetonitrile, then the ethyl 2-methylacetoacetate solution was added to the reaction flask through a funnel, and then 10mL of acetonitrile was added, and the initial no The colored reaction s...
Embodiment 2
[0063] Example 2: Evolution of CpK-specific aminoacyl-tRNA synthetases
[0064] For the site-specific insertion of CpKs in the gene, an aminoacyl-tRNA synthetase / tRNA orthogonal pair derived from Methanosarcina barkeri needs to be introduced into the E.coli host cell used Amber suppresses lysyl tRNA (Mb tRNA CUA Pyl ) / lysyl tRNA synthetase (Mb PylRS, wild type, its amino acid sequence is SEQ ID NO: 2) pair.
[0065]The Mb PylRS mutant library was constructed in the kanamycin-resistant pBK plasmid (purchased from the Peter G. Schultz laboratory of the Scripps Research Institute in the United States), and located between the promoter and terminator of E. coli glutamine synthetase on the plasmid . The synthetic enzyme mutation library used is the pBk-CpK library, and the construction method of the mutation library is: select 5 sites (L266, L270, Y271, L274 and C313) on the Mb PylRS gene, and perform random mutation by PCR . Positive and negative screens were then performed t...
Embodiment 3
[0067] Example 3: Expressing CpK-myoglobin and performing an in vitro photoclick reaction
[0068] Orthogonal tRNA (SEQ ID NO: 1) and the nucleotide sequence encoding myoglobin (4TAG) (SEQ ID NO: 5) were constructed on the pBAD vector (purchased from the laboratory of Peter G. Schultz, Scripps Research Institute, USA) , the screened nucleotide sequence (SEQ ID NO: 3) encoding CpKRS was constructed on the pBK vector (purchased from Peter G. company). Pick a single clone and grow to OD at 37°C 600 When it was approximately equal to 0.5, 1 mM CpK and 0.2% arabinose (purchased from sigma company) were added to the LB medium to culture the cells, and no CpK was added to the control. After 6-7 hours, the bacteria were harvested, and the protein was purified by Ni-NTA, and analyzed by SDS-PAGE electrophoresis ( Figure 4 a).
[0069] We found that the full-length myoglobin can only be purified in the medium with CpK, which indicates that the screened CpKRS can specifically recogn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com