A kind of preparation method of cefditoren pivoxil intermediate
A technology for cefditoren pivoxil and intermediates, which is applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as poor yield, and achieve the effects of good yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
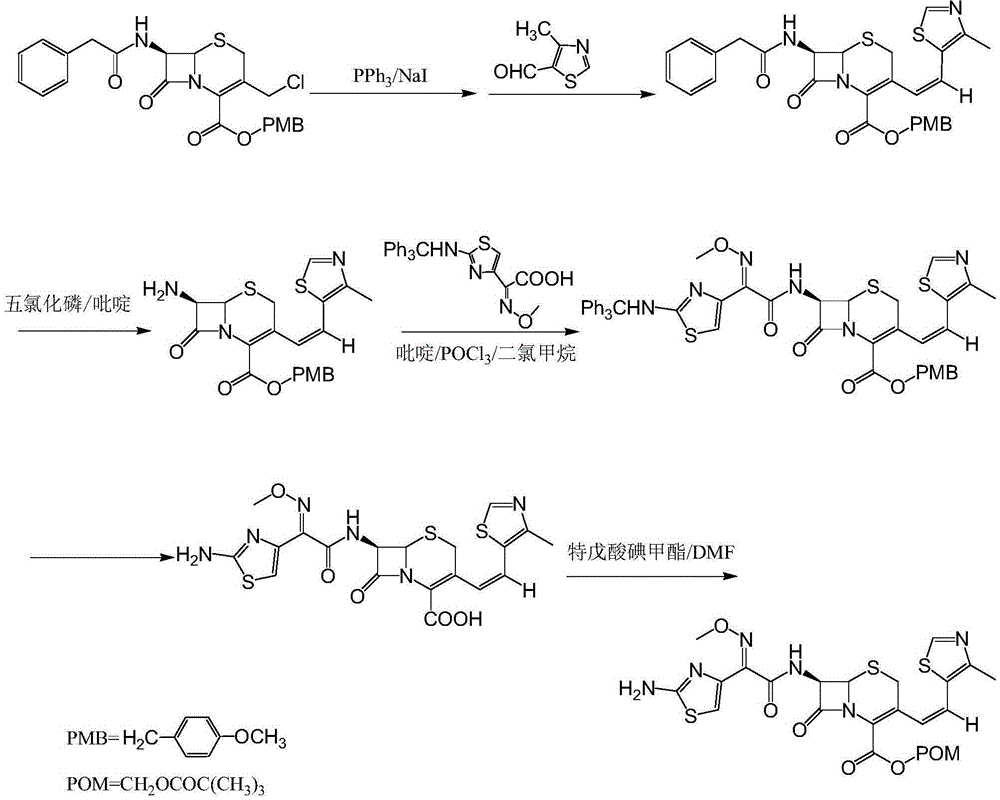
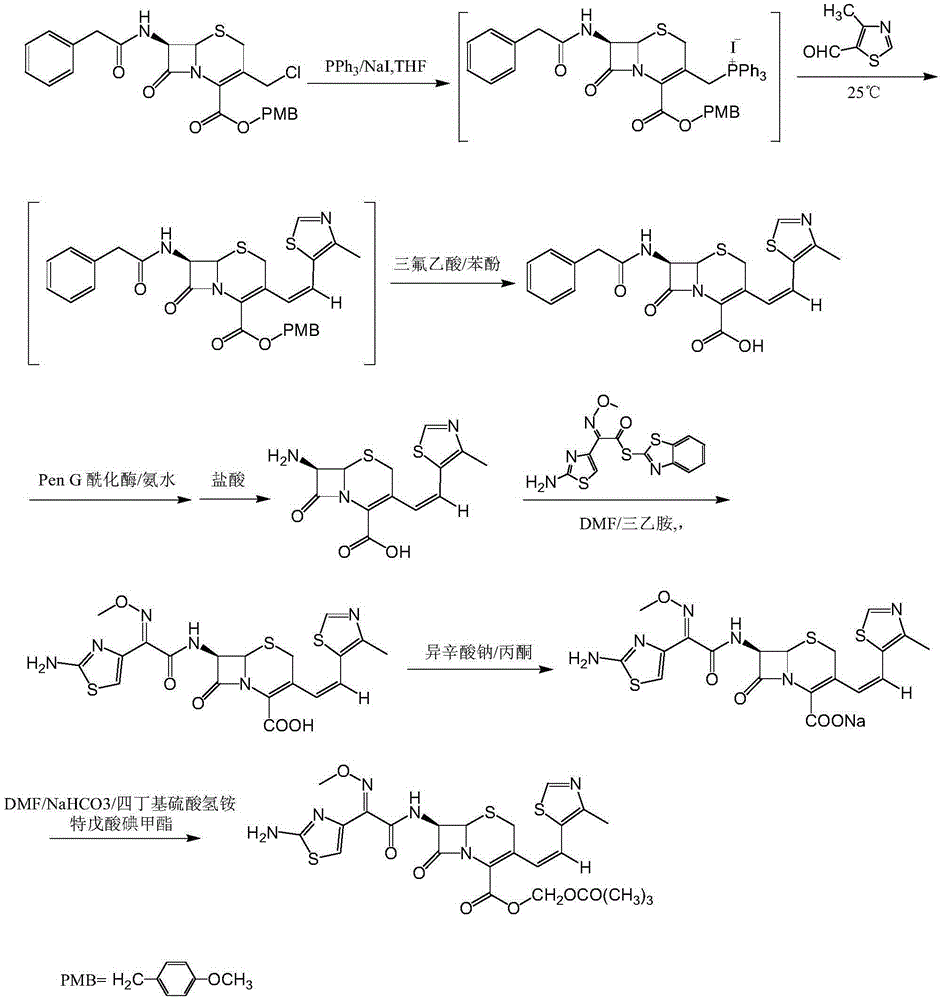
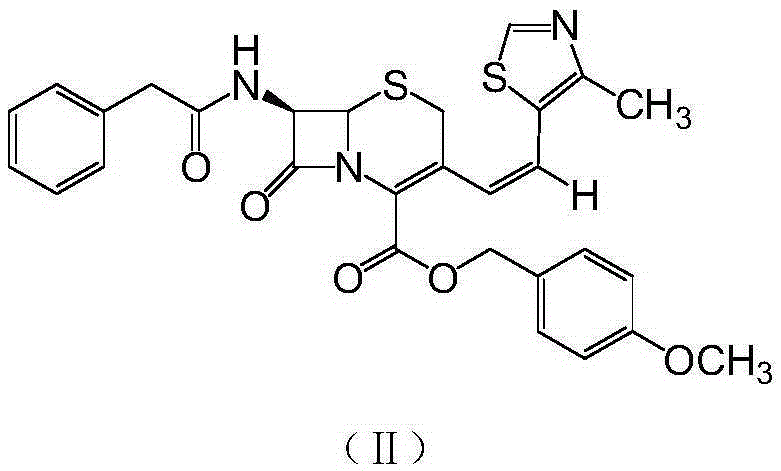
[0025] 7-Phenylacetamido-3-[(Z)-2-(4-methyl-5-thiazolyl)vinyl]-4-cephalosporanic acid p-methoxybenzyl ester
[0026] Put 100L of purified water, 100L of dichloromethane, 25kg of GCLE, 8.2kg of anhydrous sodium iodide, and 14.5kg of triphenylphosphine into the reaction kettle, and react at 30°C for 1.5 hours. When the temperature was lowered to 0°C, 2% sodium hydroxide was added dropwise to adjust the pH to 7. The layers were separated, the temperature of the dichloromethane layer was lowered to -25°C, 20 kg of 4-methyl-5-thiazole formaldehyde was added, and the reaction was kept overnight. After the reaction was complete, 150 L of 5% sodium bisulfite solution was added and stirred for half an hour. The layers were separated, the dichloromethane layer was concentrated to dryness in vacuo, 200 L of methanol was added, and the mixture was stirred at 0°C for 2 hours. After centrifugation, 27.5 kg of the title compound was obtained with a yield of 95.5%.
Embodiment 2
[0028] 7-Phenylacetamido-3-[(Z)-2-(4-methyl-5-thiazolyl)vinyl]-4-cephalosporanic acid p-methoxybenzyl ester
[0029] Put 100L of purified water, 100L of chloroform, 25kg of GCLE, 8.2kg of anhydrous sodium iodide, and 14.5kg of triphenylphosphine into the reaction kettle, and react at 30°C for 1.5 hours. When the temperature was lowered to 0°C, 2% sodium hydroxide was added dropwise to adjust the pH to 7. The layers were separated, the temperature of the dichloromethane layer was lowered to -25°C, 20 kg of 4-methyl-5-thiazole formaldehyde was added, and the reaction was kept overnight. After the reaction was complete, 150 L of 5% sodium bisulfite solution was added and stirred for half an hour. The layers were separated, the chloroform layer was concentrated to dryness in vacuo, 200 L of methanol was added, and the mixture was stirred at 0°C for 2 hours. After centrifugation, 27.2 kg of the title compound was obtained with a yield of 94.4%.
Embodiment 3
[0031] 7-Phenylacetamido-3-[(Z)-2-(4-methyl-5-thiazolyl)vinyl]-4-cephalosporanic acid p-methoxybenzyl ester
[0032] Put 100L of purified water, 100L of ethyl acetate, 25kg of GCLE, 8.2kg of anhydrous sodium iodide, and 14.5kg of triphenylphosphine into the reaction kettle, and react at 30°C for 1.5 hours. When the temperature was lowered to 0°C, 2% sodium hydroxide was added dropwise to adjust the pH to 7. The layers were separated, the temperature of the dichloromethane layer was lowered to -25°C, 20 kg of 4-methyl-5-thiazole formaldehyde was added, and the reaction was kept overnight. After the reaction was complete, 150 L of 5% sodium bisulfite solution was added and stirred for half an hour. The layers were separated, the ethyl acetate layer was concentrated to dryness in vacuo, 200 L of methanol was added, and the mixture was stirred at 0°C for 2 hours. After centrifugation, 27.7kg of the title compound was obtained with a yield of 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com