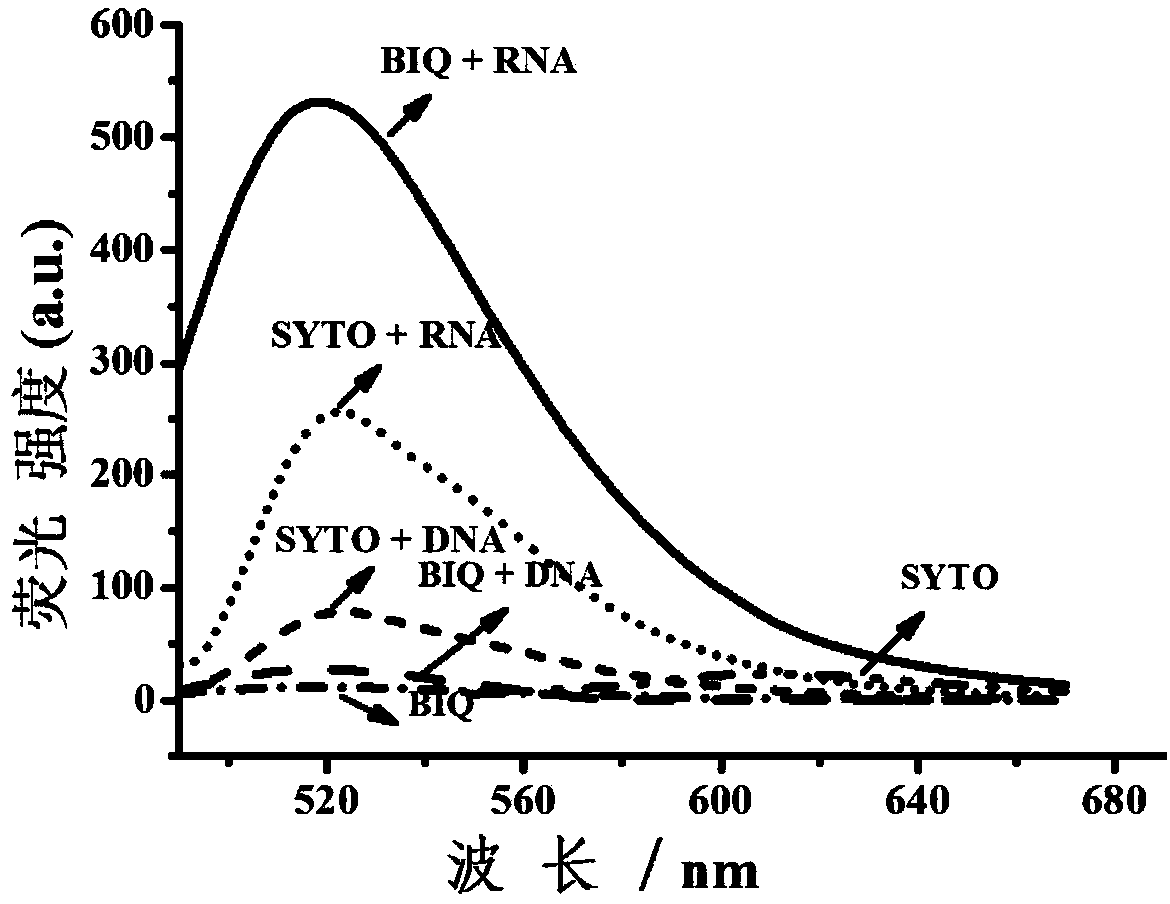
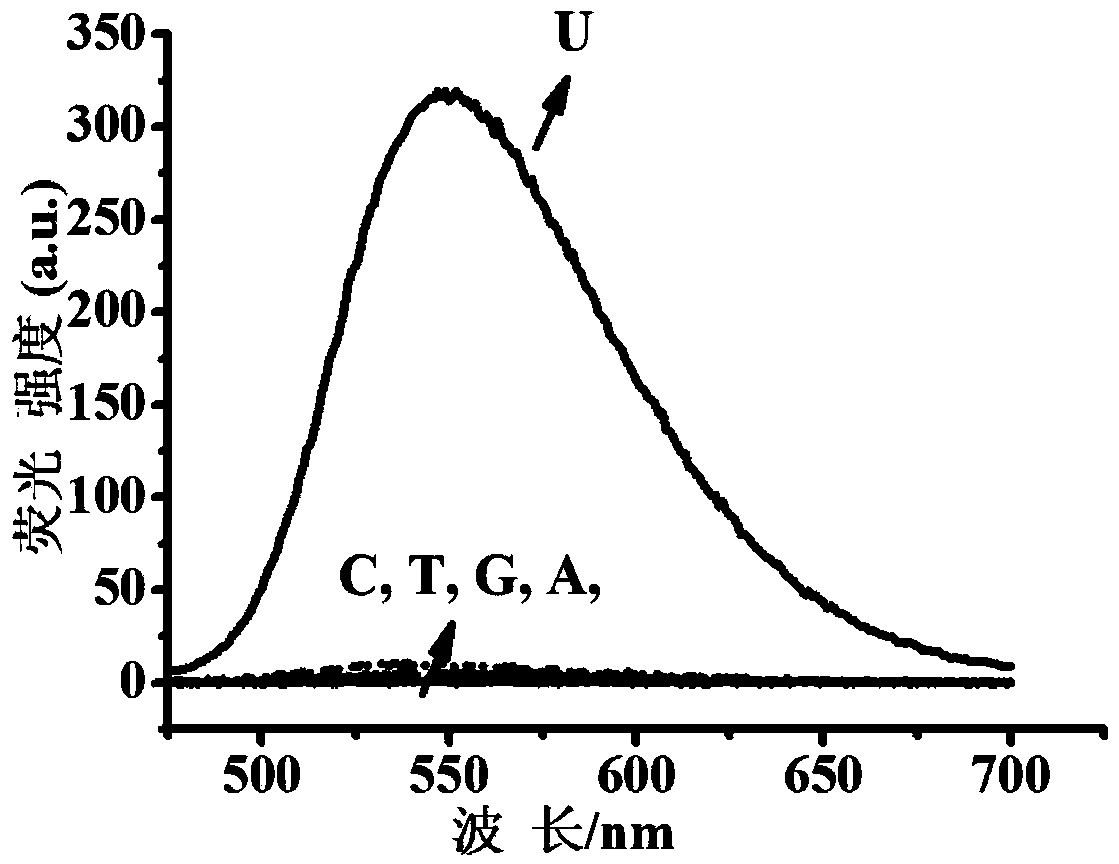
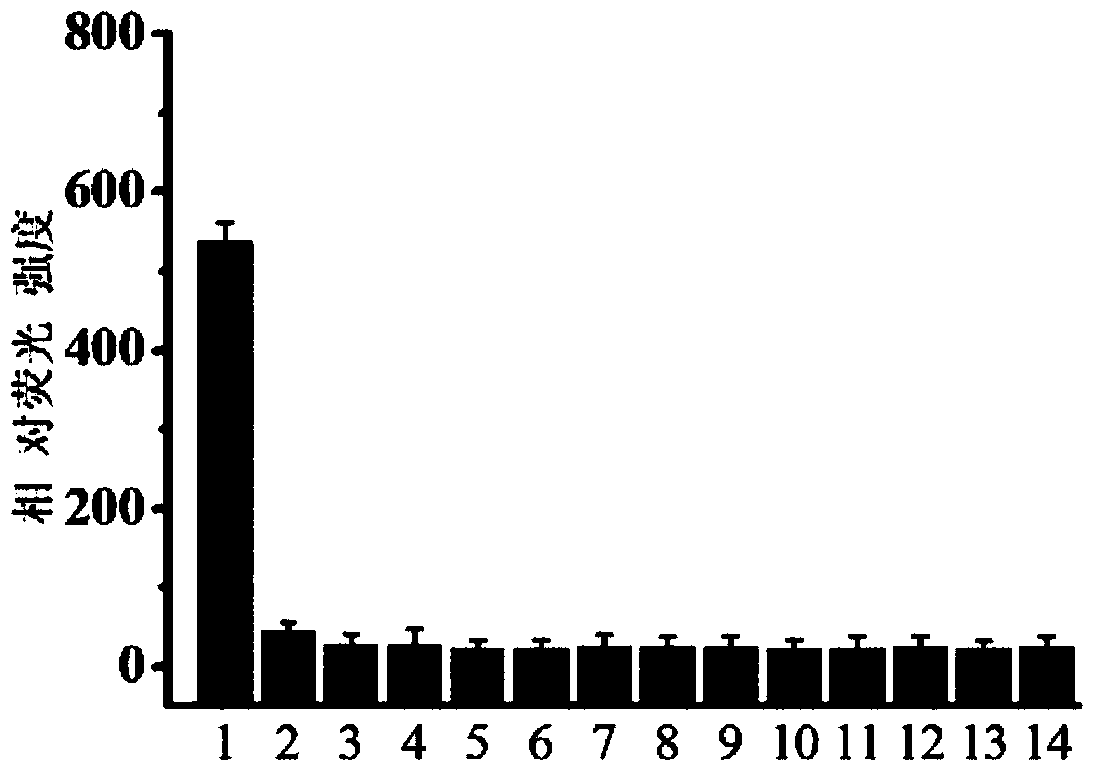
Fluorescent dye with nitrobenzimidazole as RNA (ribonucleic acid) recognition group as well as preparation method and application of fluorescent dye
A technology of nitrobenzimidazole and fluorescent dyes, which is applied in the field of fluorescent dyes, can solve the problems of low phototoxicity, biological toxicity, photobleaching, high phototoxicity, and poor photostability, and achieve good cell membrane permeability, High selectivity and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The preparation method of the fluorescent dye using nitrobenzimidazole as the RNA recognition group comprises the following steps: reacting the parent body of D with 6-nitrobenzimidazole in a molar ratio of 1:1-1:5, and reacting The temperature is 25-150°C, the reaction time is 6-48 hours, the reaction solvent is one or more of dichloromethane, ethanol and ethyl acetate, and the reaction is carried out in the presence of an organic base, with 4-dimethylamino Pyridine is the catalyst.
[0062] The fluorescent dye with nitrobenzimidazole as the RNA recognition group is a compound of general formula V, and the preparation method of the compound of general formula V comprises the following steps:
[0063]
[0064] (1), ethanolamine and concentrated hydrobromic acid according to the literature (AndrewF, DianaF, KarenLG.Drugs.1992.43(4):561:) to prepare the compound of general formula VIII
[0065]
[0066] (2), the preparation of the compound of general formula VI com...
Embodiment 1
[0090] Prepare fluorescent probe BIQ:
[0091]
[0092] (1) Synthesis of compounds of general formula VIII
[0093] Ethanolamine (122.2g, 120.8mL, 2.0mol) was added dropwise to concentrated hydrobromic acid (733mL, 5.4mol) under stirring at 10°C, and the temperature was controlled at 10-15°C. After dropping, continue stirring for 1h, and then add 400mL of xylene , warming up to reflux, and after separating about 515mL of water (containing a small amount of hydrobromic acid) with a water separator, the water pump decompresses to evaporate xylene and excess hydrobromic acid. Cool the solution to about 70%, add ice-cold (usually about 4°C) acetone (365mL), stir the mixture fully, so that the dark-colored solid is fully soaked, cool, suction filter, and cool (usually about 4°C) ) washed with acetone (150mL), sucked dry, and dried to obtain a white crystalline product of the compound of general formula VIII, weighing 358.8g, yield 87.6%, M.P.173-175°C.
[0094] (2) Synthesis o...
Embodiment 2
[0136] Preparation of Fluorescent Probe IX
[0137]
[0138] (1) Synthesis of compounds of general formula X
[0139] Dissolve 10mmol of p-fluorobenzaldehyde and 10mmol of 6-nitrobenzimidazole in 20ml of dry DMF (dimethyl fumarate), add a small amount of potassium carbonate (catalyst) to the mixture each time, and The total amount of potassium was 12 mmol, and the addition was completed within 15 minutes. Reacted under stirring at 110°C for 10-12h. After the reaction was completed, the reaction system was cooled to room temperature, filtered with suction, and the filtrate was extracted with ethyl acetate. The organic phase was washed 3 times with water, dried over anhydrous sodium sulfate, and concentrated to obtain an oil. The product was separated by column chromatography.
[0140] (2) Synthesis of fluorescent dye with nitrobenzimidazole as RNA recognition group (referred to as fluorescent probe IX)
[0141] Put 0.85mmol of the intermediate compound of general formul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com