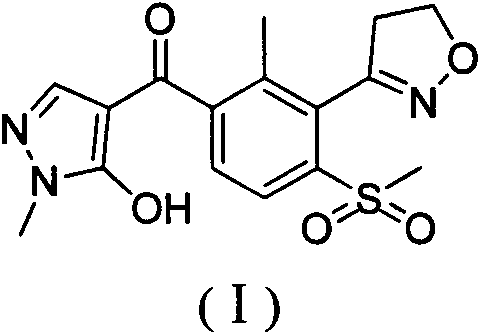
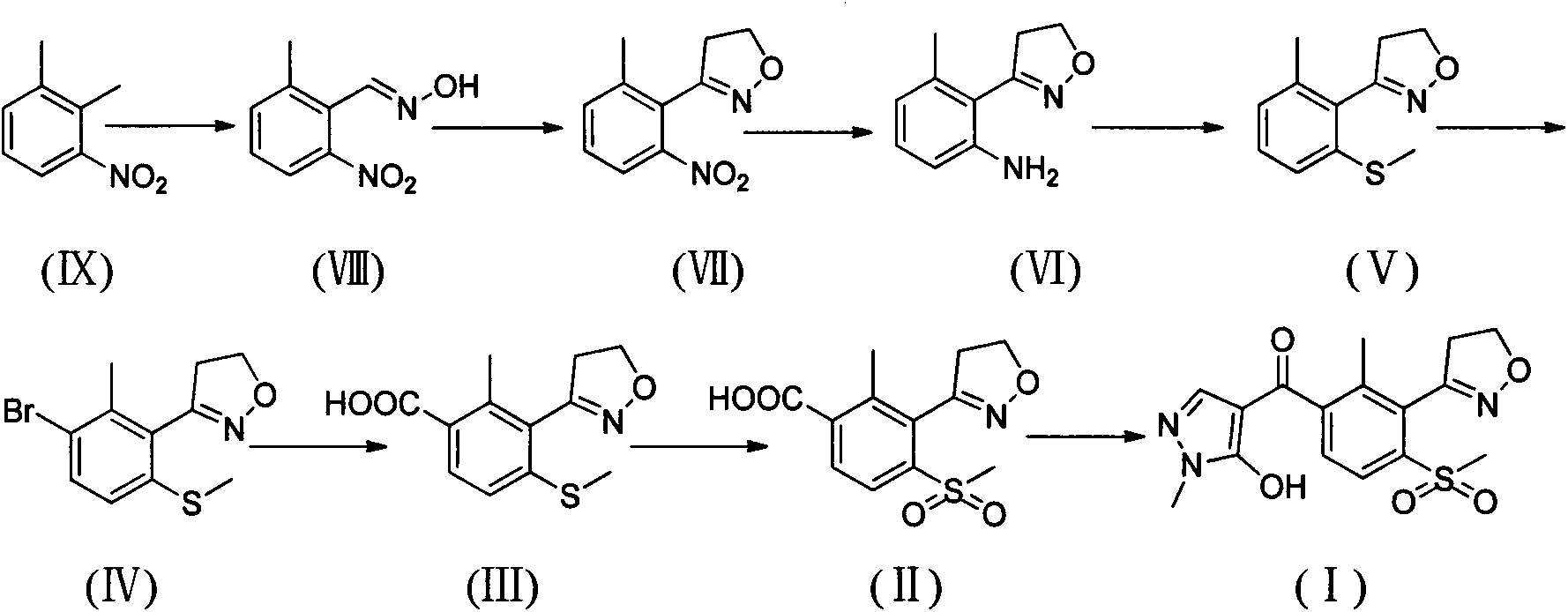
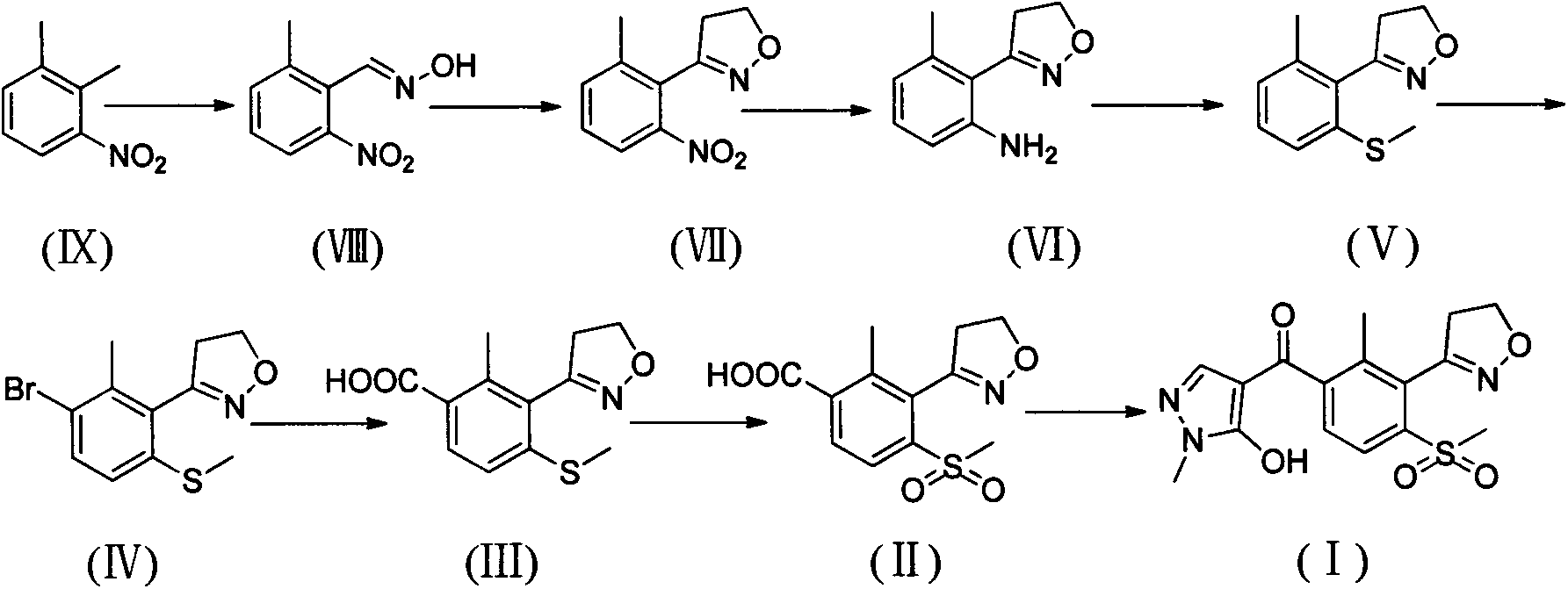
Method for preparing herbicide topramezone
A technology based on phenyl and isoxazolyl, which is applied in the field of preparing the herbicide fenflumezone, which can solve the problems of difficult recovery, expensive catalyst, and low total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Synthesis of compound (VIII)
[0060]
[0061] Add 120ml of N,N-dimethylformamide into a 500ml reaction flask, cool to -40°C, slowly add 33.6g of potassium methoxide in batches, and stir evenly. Then, under strong stirring, 30g (0.2mol) of 3-nitro-o-xylene and 27.4g of n-butyl nitrite mixed solution was slowly added dropwise to the reaction solution, and the temperature was controlled at -45~-35°C for about 4.5h. After the dropwise addition, the reaction was continued at this temperature for 3.5h. After the reaction is completed, slowly add 40mL of water dropwise, then add 40mL of glacial acetic acid to adjust the pH of the reaction solution to 5-6, control the temperature below -5°C, then pour the reaction solution into 600ml of ice water, stir for 30min, filter with suction, and wash the filter cake with water , and dried to obtain 30.9 g of off-white solid. The crude product was added to 100ml of toluene, stirred at room temperature for 1.5h, filtered, and the f...
Embodiment 2
[0064] Synthesis of compound (VII)
[0065]
[0066] Add 10.8g (0.06mol) of 2-methyl-6-nitrobenzaldehyde oxime to a 250ml reaction bottle, dissolve it in 100ml of acetonitrile, heat to 60°C, add a small amount of N-chlorosuccinimide, wait until the reaction starts, Cool the reaction solution, dissolve 8.2g of N-chlorosuccinimide in 60ml of acetonitrile and slowly add it dropwise into the reaction solution at 40-50°C, continue stirring for 30min after the addition, evaporate the acetonitrile under reduced pressure, and wash the residue with 150ml of toluene After dissolving, stir for 1 hour, filter with suction, transfer the filtrate to a 250ml autoclave, slowly add 6.0g of triethylamine dropwise, and then feed ethylene to react for 8 hours under a pressure of 6bar. After the reaction, the reaction liquid was washed with saturated sodium bicarbonate solution and water respectively, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to ...
Embodiment 3
[0069] Synthesis of compound (VI)
[0070]
[0071] Add 10.0g (0.05mol) 3-(2-methyl-6-nitrophenyl)-4,5-dihydroisoxazole in 250ml reaction bottle, 35g stannous chloride dihydrate, dissolve with 150ml ethanol, Heat to reflux for 5 hours, spin to dry ethanol, adjust the pH of the residue to 10-11 with 20% sodium hydroxide, add ethyl acetate, stir well, filter with suction, wash the filter cake with ethyl acetate, and wash the filtrate three times with water, without After drying with sodium sulfate, the solvent was distilled off under reduced pressure to obtain 7.8 g of brown-gray crystalline product with a yield of 91.3%.
[0072] 1 H NMR (CDCl 3 , 300Hz) δ: 2.27(s, 3H, CH 3 ), 3.26(t, 2H, CH 2 ), 4.10 (s, 2H, NH 2 ), 4.46(t, 2H, CH 2 ), 6.61(t, 2H, Ar-H), 7.06(t, 1H, Ar-H); ESI-MS: 199.1[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com