Catalyst for preparing ethylbenzene by reacting ethylene with benzene, and preparation method and application of catalyst
A catalyst and ethylene technology, applied in the direction of molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of gas escape, increased production costs, and high equipment requirements, so as to reduce equipment requirements, save production costs, The effect of lowering the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The preparation method of the above catalyst is described in detail below.
[0051] The method for preparing the catalyst used for the reaction of benzene and ethylene to prepare ethylbenzene is as follows:
[0052](1) At 25°C, immerse the ZSM-5 molecular sieve in the rare earth element metal salt solution for 2-10h for exchange, and filter after the exchange; then dry the filtered molecular sieve at 60°C-120°C for 2-10h, then Roast at 400-600°C for 3-10 hours to obtain modified ZSM-5 molecular sieve.
[0053] (2) At 25°C, impregnate ultra-stable Y (USY) molecular sieves with rare earth element metal salt solution for 2-10 hours, then bake at 100-120°C for 2-10 hours, and then bake at 400-600°C for 3-10 hours ; Then impregnate the USY molecular sieve loaded with rare earth element oxides in the nitrate solution of alkaline earth metal for 2-10 hours at 25°C, then bake at 100-120°C for 2-10 hours, and then dry at 400-600°C ℃ calcination for 3-10 hours to obtain the mod...
Embodiment 1
[0064] This example is used to illustrate the method for preparing modified ZSM-5 molecular sieve and modified USY molecular sieve.
[0065] Take 1500 grams of commercially available ZSM-5 molecular sieves with a silicon-aluminum ratio of 60 (commercially available, finished molecular sieves produced by Nankai University), and exchange them with 200 mL of a solution containing 20 grams of lanthanum nitrate, that is, at 25 ° C, rapidly dissolve in the lanthanum nitrate solution Stir and exchange for 2 hours, then filter, dry the filtered molecular sieve at 60°C for 10 hours, then roast at 500°C for 4 hours, and after crushing, a modified ZSM-5 molecular sieve a, which contains rare earth lanthanum oxide and rare earth element lanthanum The content is 0.1%.
[0066] Weigh 2000 grams of commercially available NaY molecular sieves with a silicon-aluminum ratio of 4, exchange NaY molecular sieves with 10% H2SiF6 solution at 95°C for 6 hours, filter, dry the filter cake at 120°C for...
Embodiment 2
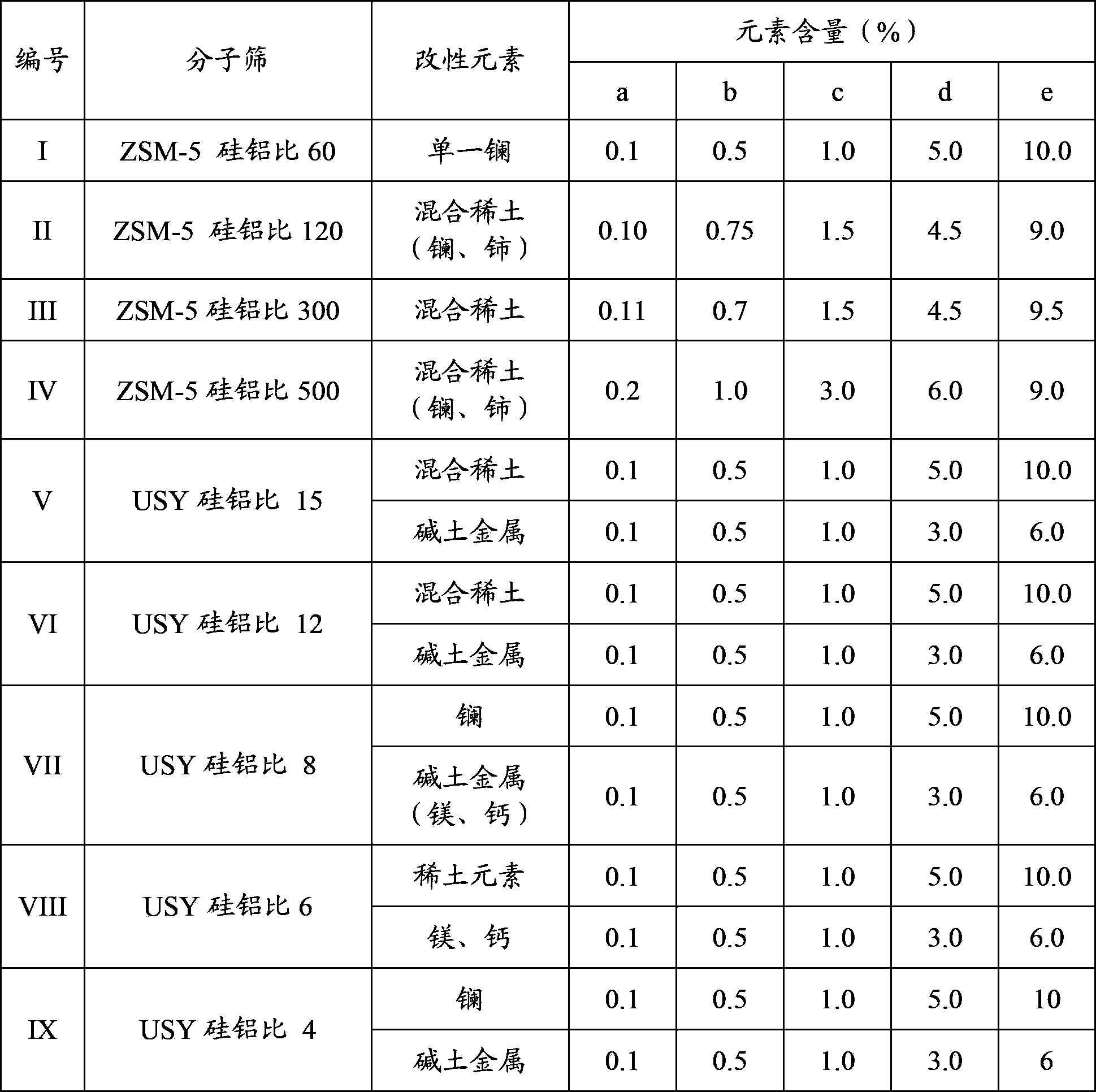
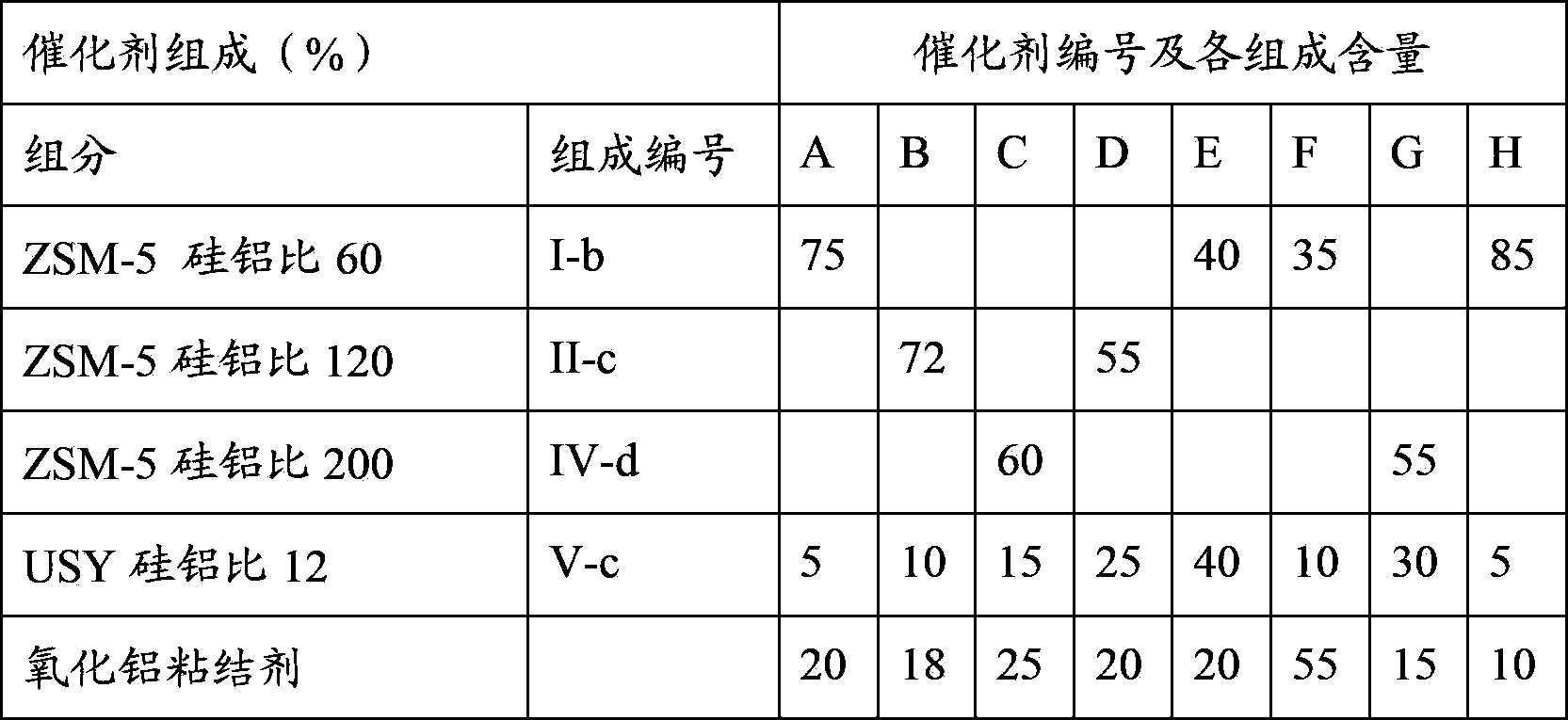
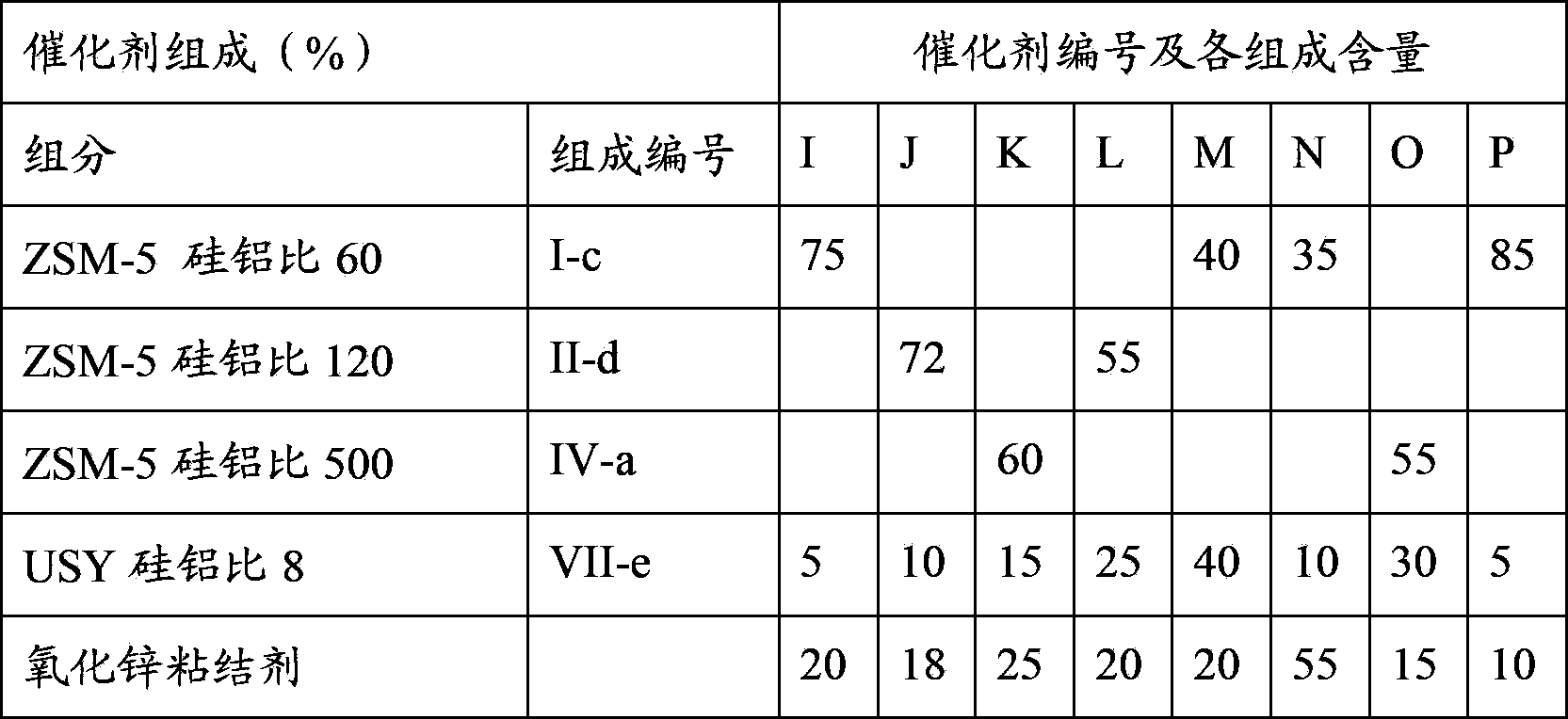
[0069] According to the molecular sieve modification method in Example 1 and the various modification conditions mentioned in the present invention, with the composition requirements in Table 1, the various ZSM-5 and USY molecular sieves in Table 1 were modified respectively. The obtained modified molecular sieves are numbered I-a, I-b, I-c, I-d, I-e, II-a, II-b, II-c, II-d, III-e....
[0070] Table 1 Molecular sieve modification element content requirements
[0071]
[0072] Note: Miscellaneous rare earths come from rare earth chlorides or rare earth nitrates, mainly containing lanthanum, cerium, neodymium and praseodymium; lanthanum comes from lanthanum nitrate; alkaline earth metals come from nitrates of alkaline earth metal elements, or a mixture of magnesium nitrate, beryllium nitrate or calcium nitrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com