Hybrid azaguanosine as well as synthesis method and application thereof in DNA sequencing
A technology of guanine nucleoside and guanine nucleotide, which is applied in the fields of chemical synthesis and biochemistry, and can solve the problems of inapplicability to large-scale production, low yield, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
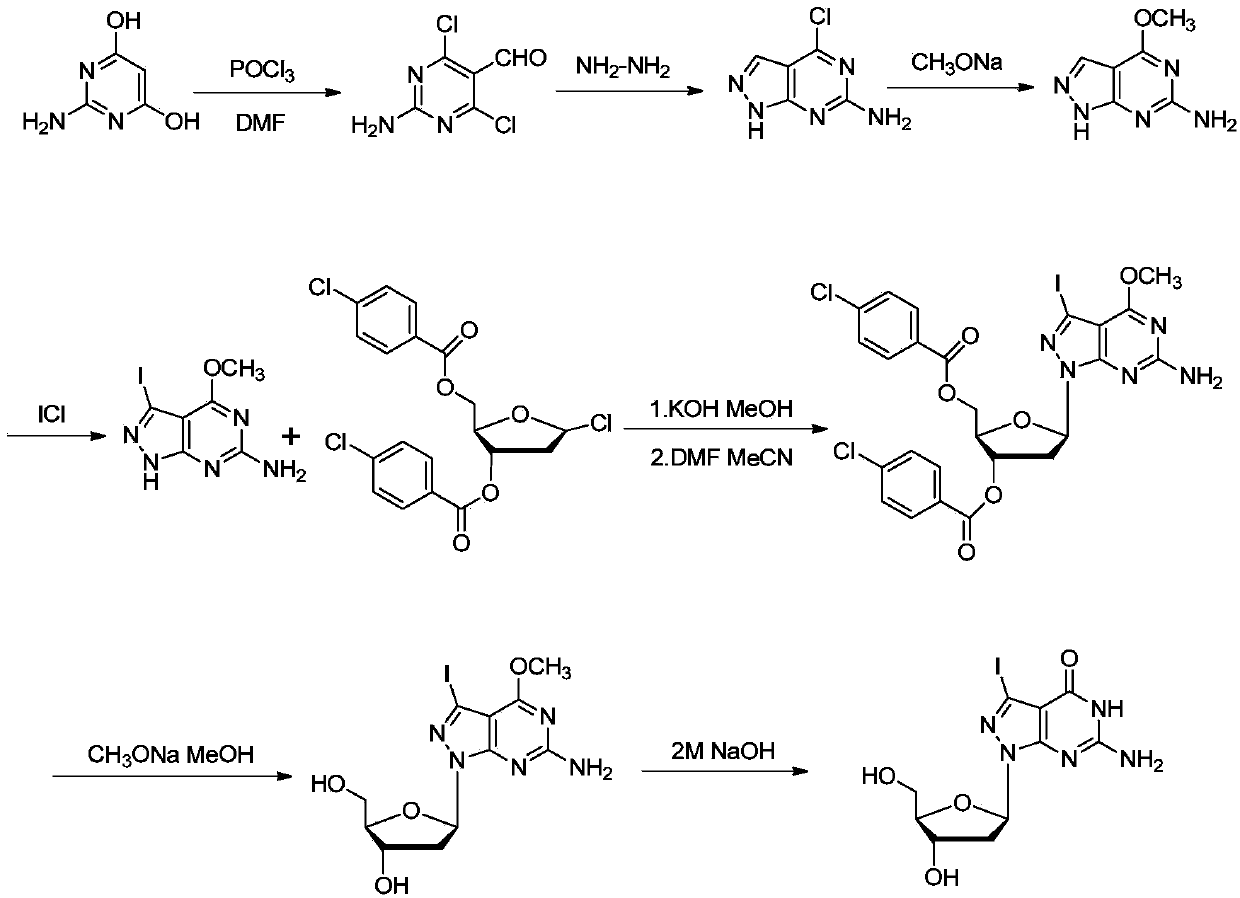
[0076] The synthetic method of embodiment 1,7-deaza-7-iodo-8-aza-2'-deoxyguanosine
[0077] The synthesizing scheme of formula (I1-iodine) compound (i.e. 7-deaza-7-halogen-8-aza-guanosine) in this example is as follows figure 1 As shown, the specific synthetic method comprises the following steps respectively:
[0078] step one,
[0079]
[0080] Dissolve 2-amino-4,6-dihydroxypyrimidine (12.5 g, 100 mmol) in 75 mL POCl 3 and 17.5mL of anhydrous DMF, stirred at room temperature for 10min, under nitrogen protection, refluxed at 105°C for 1.5h, stopped heating, cooled to room temperature, spin out the solvent, dissolved the solid in 2L of ice water, stirred at 50°C for 2h Filter, wash with water and ethyl acetate, and dry in vacuo to obtain 11.0 g of light yellow solid with a yield of 57.6%.
[0081] 1 H NMR (400MHz, DMSO): δ = 10.05 (s, 1H, -CHO), 8.49 (brs, 2H, -NH 2 ).
[0082] Step two,
[0083]
[0084] The formula (Ⅷ) compound (2.4g, 12.5mmol) was added into ...
Embodiment 2、7
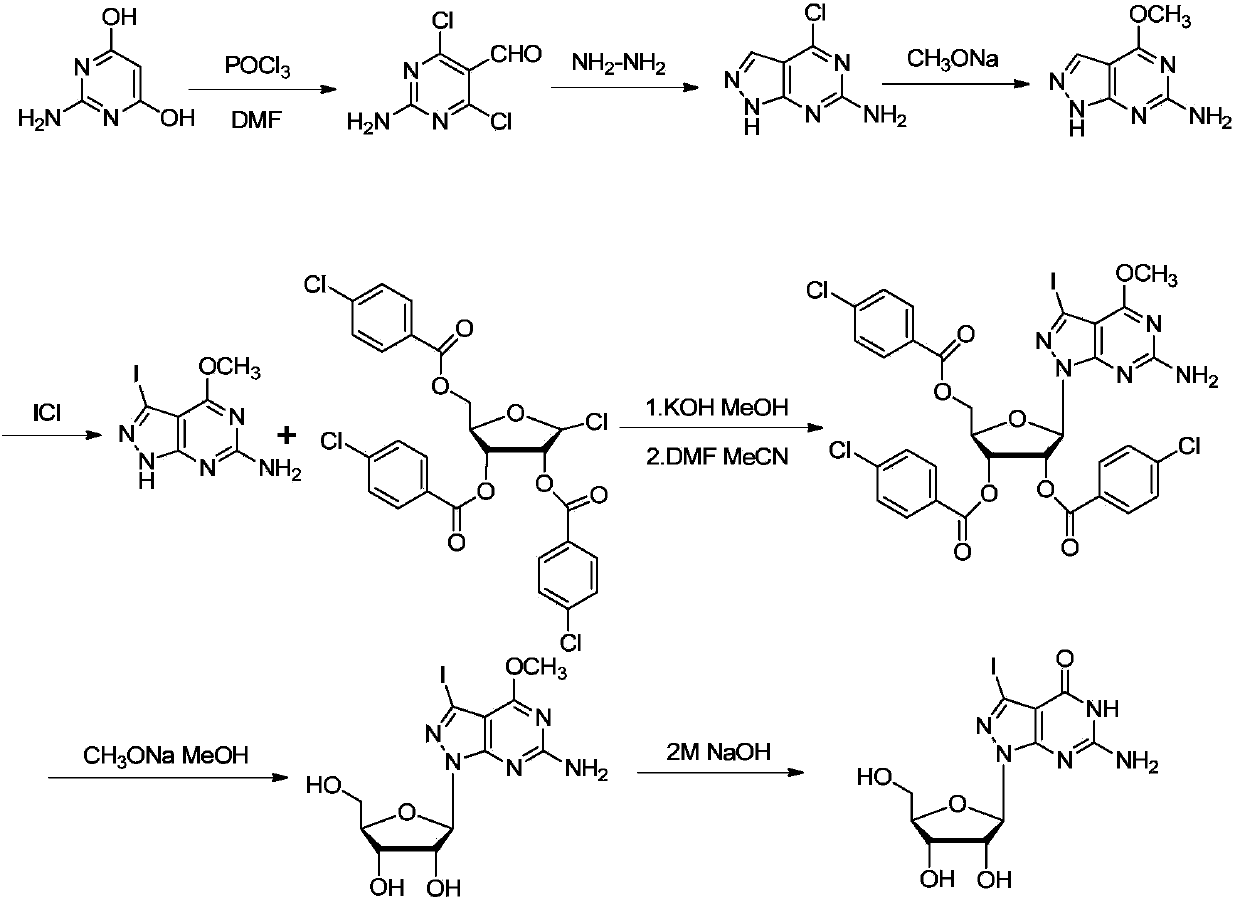
[0107] The synthetic method of embodiment 2,7-deaza-7-iodo-8-aza-guanosine
[0108] The synthetic schematic diagram of (I2-iodine) in the present embodiment is as follows figure 2 As shown, the specific synthetic method comprises the following steps respectively:
[0109] Step 1, when compound VI, R 3 for , the specific reaction formula is as follows:
[0110] (Ⅲ2-iodine)
[0111] Add the compound of formula (Ⅳ-iodine) (879 mg, 3.02 mmol) into 3 mL of anhydrous methanol, then add 1.25 mL of 2.85 M potassium hydroxide / methanol solution, stir at room temperature for 1 min, add 3 mL of toluene, spin out the solvent, and dry the solid in vacuum After that, add 3.5mL of anhydrous acetonitrile and 1.5mL of anhydrous DMF mixed solution, nitrogen protection, add 1-chloro 2,3,5-di-p-chlorobenzoyloxy-2-deoxy-D-ribofuranose (1.79 g, 3.06mmol), after stirring at room temperature for 24h, spin out the solvent, column chromatography [V (petroleum ether): V (ethyl acetate) = 5: 1]...
Embodiment 3
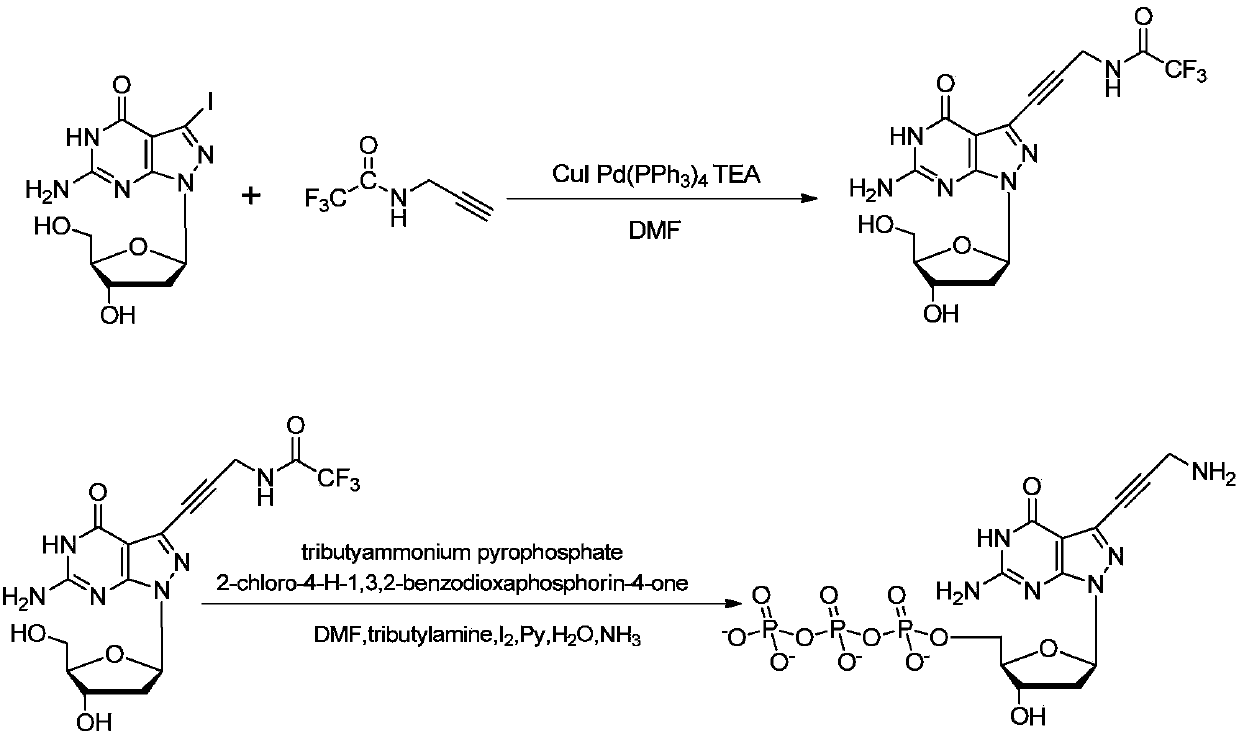
[0122] Example 3, 7-deaza-7-iodo-8-aza-2'-deoxyguanosine in the synthesis of dG(8-aza)TP(AP 3 ) used in way
[0123] In this example, dG(8-aza)TP(AP 3 ) Synthesis schematic diagram as image 3 As shown, the specific synthetic method comprises the following steps respectively:
[0124] step one,
[0125]
[0126] Add formula (I 1-iodine) compound (78.6mg, 0.2mmol) in a single-necked bottle, then weigh CuI (9.5mg, 0.5mmol) and Pd (PPh 3 ) 4 (23.1mg, 0.02mmol) was added to the reaction flask, evacuated, nitrogen protected, wrapped in aluminum foil, added 5mL of anhydrous DMF, stirred to dissolve, injected with TEA (40.4mg, 0.4mmol) and trifluoroacetylpropargylamine (90.6mg, 0.6mmol), stirred at 50°C for 12 hours, the reaction was completed, the solvent was spun out, the residue was dissolved in 50mL ethyl acetate, washed with saturated sodium bicarbonate solution and saturated sodium chloride solution successively, dried over anhydrous sodium sulfate, concentrated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com