Preparation method of precursor of lithium iron phosphate-lithium vanadium phosphate composite
A technology of lithium vanadium phosphate and composite materials, which is applied in the direction of phosphorus compounds, iron compounds, chemical instruments and methods, etc., can solve the problems such as the electronic conductivity of lithium iron phosphate-lithium vanadium phosphate composite cathode materials needs to be improved, and achieve low cost, The process is simple and the product quality is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
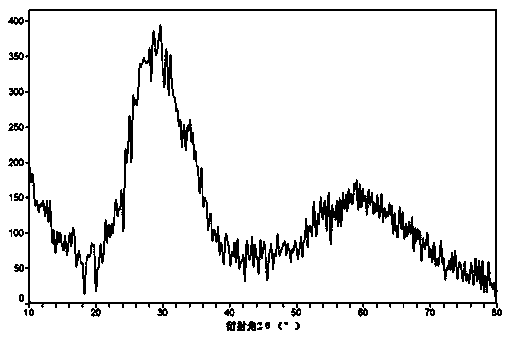
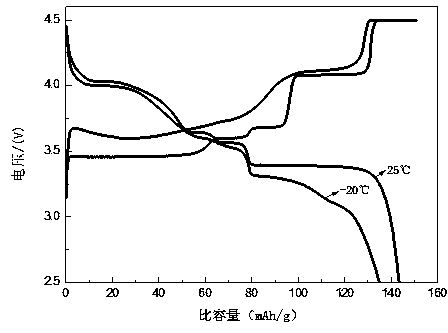
Image
Examples
Embodiment 1
[0018] This embodiment includes the following steps:
[0019] (1) 85.5g of graphene oxide suspension with a mass fraction of 2% was placed in a 5L stirred reaction kettle with an ultrasonic device, and ultrasonically treated for 0.5h under the condition of 30KHz;
[0020] (2) 39.9g of ferric sulfate was added to 1L of deionized water to prepare a ferric sulfate solution with a concentration of 0.1mol / L, and 36.8g of sodium orthovanadate was added to 1L of deionized water to prepare a concentration of 0.2mol / L of ferric sulfate. Sodium vanadate solution; then simultaneously add the prepared ferric sulfate solution and sodium orthovanadate solution into the stirring reaction kettle at a speed of 400mL / h, control the stirring speed to be 200rpm, adjust the pH to 6 with ammonia water, and react for 1.0h;
[0021] (3) Add 3.0g polyaniline to refine the particle size of iron vanadate particles, then stir at 200rpm for 0.5h, age for 3h, filter, wash, and blow dry at 100°C for 5h to o...
Embodiment 2
[0027] This embodiment includes the following steps:
[0028] (1) 136.8g of graphene oxide suspension with a mass fraction of 2% was placed in a 5L stirring reaction kettle with an ultrasonic device, and ultrasonically treated for 0.2h under the condition of 20KHz;
[0029] (2) 31.99g of ferric sulfate was added to 1L of deionized water to prepare a ferric sulfate solution with a concentration of 0.08mol / L, and 29.44g of sodium orthovanadate was added to 1L of deionized water to prepare a concentration of 0.16mol / L of ferric sulfate. Sodium vanadate solution; then simultaneously add the prepared ferric sulfate solution and sodium orthovanadate solution into the stirring reaction kettle at a speed of 200mL / h, control the stirring speed to be 50rpm, adjust the pH to 2 with ammonia water, and react for 1h;
[0030] (3) 3.5g of polyaniline was added, then stirred at 200rpm for 0.5h, aged for 2h, filtered, washed, and freeze-dried at -50°C for 20h to obtain 30.36g of lithium iron p...
Embodiment 3
[0034] This embodiment includes the following steps:
[0035] (1) 90.8g of graphene oxide suspension with a mass fraction of 2% was placed in a 5L stirring reaction kettle with an ultrasonic device, and ultrasonically treated at 40KHz for 2h;
[0036] (2) 47.98g of ferric sulfate was added to 1L of deionized water to prepare a ferric sulfate solution with a concentration of 0.12mol / L, and 44.16g of sodium orthovanadate was added to 1L of deionized water to prepare a concentration of 0.24mol / L of ferric sulfate. Sodium vanadate solution; then simultaneously add the prepared ferric sulfate solution and sodium orthovanadate solution into the reaction kettle at a speed of 600mL / h, control the stirring speed to be 400rpm, adjust the pH to 8 with ammonia water, and react for 4h;
[0037] (3) 3.8g of polyaniline was added, then stirred at 200rpm for 0.5h, aged for 4h, filtered, washed, and dried at 90°C and -0.1MPa for 8h to obtain 45.34g of lithium iron phosphate-lithium vanadium phos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com