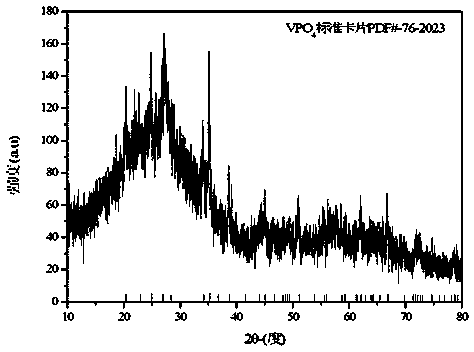
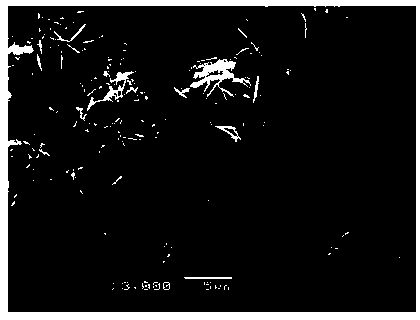
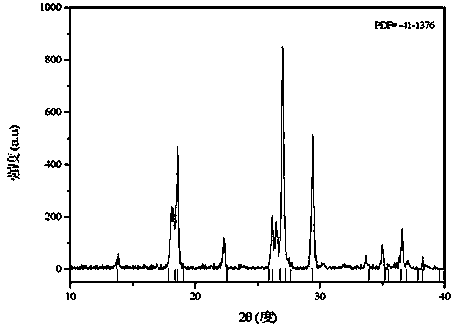
Preparation method for nano sheet-shaped lithium ion battery positive electrode material fluorine lithium vanadium phosphate
A lithium-ion battery, lithium vanadium fluorophosphate technology, applied in battery electrodes, nanotechnology, nanotechnology and other directions, can solve the problems of low electronic conductivity, limit high-rate discharge performance, etc., and achieve high specific surface area and excellent electrochemical performance. , is conducive to the effect of embedding and detachment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 0.91 g of vanadium pentoxide, 1.15 g of diammonium hydrogen phosphate, and 1.4 g of citric acid, dissolve them in 50 mL of deionized water; stir in a water bath at 80°C for 4 hours to form a uniform blue solution; adjust the pH of the solution to 7 with ammonia water ; Transfer the solution to a polytetrafluoroethylene tank, place the polytetrafluoroethylene tank containing the solution in a pyrolysis tank, heat and react at 250°C for 20h, cool naturally to room temperature, and take out the reaction product; filter, and filter the product Dry in a vacuum oven at 80°C to obtain an amorphous vanadium phosphate precursor; grind the dried amorphous vanadium phosphate precursor in an agate mortar, then place it in a tubular sintering furnace, sintering at 700°C for 6 h, and then naturally cooled to room temperature to obtain a crystalline vanadium phosphate precursor; weigh 0.438 g of crystalline vanadium phosphate precursor and 0.078 g of lithium fluoride, and grind t...
Embodiment 2
[0038] Weigh 1.82g of vanadium pentoxide, 2.3g of diammonium hydrogen phosphate, and 4.4g of oxalic acid, and dissolve them in 80mL of deionized water; stir in a water bath at 80°C for 2 hours to form a uniform green solution; adjust the pH of the solution to 2 with ammonia water; The solution was transferred to a polytetrafluoroethylene tank, and the polytetrafluoroethylene tank containing the solution was placed in a pyrolysis tank, heated and reacted at 280°C for 18 hours, cooled to room temperature, and the reaction product was taken out; filtered, and the filtered product was placed in a vacuum oven Dry at 80°C to obtain an amorphous vanadium phosphate precursor; grind the amorphous vanadium phosphate precursor in an agate mortar, then place it in a tube sintering furnace, and sinter at 600°C for 2 hours in an argon atmosphere , and then naturally cooled to room temperature to obtain a crystalline vanadium phosphate precursor; weigh 0.438g of vanadium phosphate precursor, ...
Embodiment 3
[0041] Weigh 1.17g of ammonium metavanadate, 1.15g of diammonium hydrogen phosphate, and 1.4g of citric acid, and dissolve them in 80mL of deionized water; stir in a water bath at 80°C for 6 hours to form a uniform green solution; adjust the pH of the solution to 12 with ammonia water; Transfer the solution to a polytetrafluoroethylene tank, place the polytetrafluoroethylene tank containing the solution in a pyrolysis tank, heat and react at 230°C for 25 hours, cool to room temperature, and take out the reaction product; filter, and put the filtered product in a vacuum Dry in an oven at 80°C to obtain an amorphous vanadium phosphate precursor; grind the amorphous vanadium phosphate precursor in an agate mortar, then place it in a tube sintering furnace, and sinter it at 725°C under an argon atmosphere 8h, then cool down to room temperature naturally to obtain the vanadium phosphate precursor; weigh 0.438g of the vanadium phosphate precursor, 0.126g of sodium fluoride, and 0.11g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com