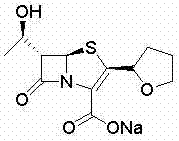
Method for synthesizing faropenem sodium
A technology of faropenem sodium and p-nitrobenzyl ester, applied in the field of medicinal chemistry, can solve the problems of difficult removal of triphenylphosphine, lower yield, expensive silver nitrate, etc., to facilitate industrial production and improve operability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (R) -tetrahydrofuran-2-thiocarboxylic acid (198g, 1.5 mol) was placed in a 3L reaction flask, and 1 mol / L sodium hydroxide bath solution (1.5 L) was added to adjust the pH to 9-10, 0- At 5°C, add dropwise 4AA (287g, 1.0mol) in acetone (1 L) solution, after the drop is complete, adjust the pH to about 8 with 1 mol / L sodium oxide, and react at room temperature for 2 h. Add water (500 ml) Dilute, extract with ethyl acetate (600 ml x 3), combine organic layers, wash with 5% sodium bicarbonate solution (300 ml x 2) and water (300 ml x 2) successively, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate , to obtain a light yellow oil (about 360 g), which was directly put into the next reaction.
Embodiment 2
[0033] The concentrated solution obtained above, triethylamine (170g, 1.7 mol) and dichloromethane (1.5 L) were mixed, and p-nitrobenzyl chlorooxalate (414.1 g, 1.7 mol) was added dropwise at 0-5 ℃, and the dropwise , react at the same temperature for 2 h, dilute with water (1 L), extract with dichloromethane (500 ml x 4), combine the organic layers, and successively add water (300 ml x 2) and 5% sodium bicarbonate solution (300 ml x 2) Wash, dry over anhydrous sodium sulfate, filter, and concentrate to obtain a light yellow oil (about 530 g), which is directly put into the next reaction.
Embodiment 3
[0035] Mix the oil obtained above, xylene (4L) and triethyl phosphite (500ml), heat to reflux for 5h, evaporate xylene and triethyl phosphite under reduced pressure, and wash the residue with ethyl acetate-n-hexane ( 1:5, 1 L) and recrystallized to obtain a light yellow solid (334.3g, 61%, based on 4AA).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com