Recombined alkaline pectinase with high pH stability and specific enzyme activity and construction method thereof
A technology of pectinase and specific enzyme activity, which is applied in the field of biogenetic engineering and can solve the problems of time-consuming, high cost, and large water consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
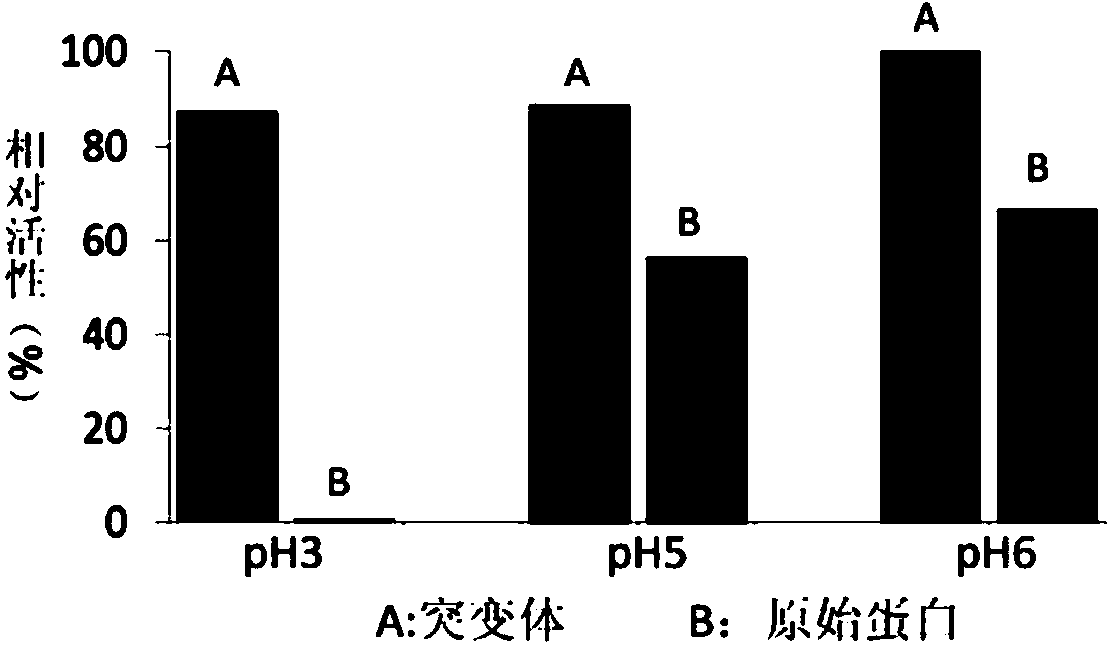
[0059] A kind of alkaline pectinase with good pH stability and high specific enzyme activity and its encoding gene of the present invention is characterized in that the pectinase gene pel168 contained in GenBank accession number AL009126 corresponds to the amino acid sequence of pectinase PEL168 The accession number is CAB12585.1, design point mutation primers (FP and RP) for site-directed mutagenesis, change the guanine G at position 457 of the published gene into thymine T, and obtain pel168V132F, making it encode the amino acid sequence of the mature peptide Valine V (GTC) at position 132 was mutated into phenylalanine F (TTC) to obtain recombinant alkaline pectinase PEL168V132F.
[0060] The recombinant alkaline pectinase of the present invention and the construction method of its encoding gene,
[0061] Including the following steps:
[0062] 1) PCR method was used to clone the gene pel168 encoding alkaline pectinase;
[0063] 2) Link the alkaline pectinase gene pel168 ...
Embodiment 2
[0076] Medium: LB resistant medium: tryptone 10 g / L, yeast extract 5 g / L, NaCl 10 g / L, kanamycin 50 mg / mL;
[0077] Cultivation method: After activating the recombinant strain Rb(DE3)-pET28a-pel168V132F of Example 1 on the LB resistance plate to prepare seeds, transfer it to liquid LB medium at an inoculum size of 1:100, at 37°C, 200 rpm cultured to OD 600 0.6, add the inducer IPTG, and then culture at 18°C, 200rpm for 14-16h.
[0078] The induced bacterial cells were ultrasonically destructed, passed through a HIS column and an ion exchange column for purification and concentration, and a purified pectinase mutant PEL168V132F was obtained.
Embodiment 3
[0080] Alkaline pectinase enzyme activity assay method: take 20 μl of diluted enzyme solution, add 2 ml of 0.2% polygalacturonic acid solution, mix well, and start the enzymatic reaction; The reaction was terminated by mol / L phosphoric acid, and the absorbance value was measured at 235 nm. The blank control is an inactive enzyme solution reaction (ie: first add 3ml of phosphoric acid and mix with the enzyme solution to be tested, then add the substrate to react for 15 minutes). The production of 1 mmol / L reducing sugar per minute is regarded as 1 enzyme activity unit (U).
[0081] Enzyme activity calculation: OD 235×10 6 × enzyme dilution factor × total reaction volume
[0082] ______________________________________
[0083] 10 3 ×t×4600×b×volume of enzyme
[0084] Where: 4600 (L.mol -1 cm -1-- )—Molar absorptivity of unsaturated polygalacturonic acid at 235 nm
[0085] t--(min)—enzymatic reaction time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com