Organic phospholipid hydrolase, gene, recombinant expression transformant and application thereof
A technology of organophosphorus ester and hydrolase, which is applied in the field of bioengineering, can solve the problems of low organophosphorus ester hydrolase activity and poor thermal stability, and achieve excellent thermal stability, high thermal stability, and high organophosphorus hydrolysis active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Screening and Gene Cloning of Organophospholipid Hydrolase PoOPH
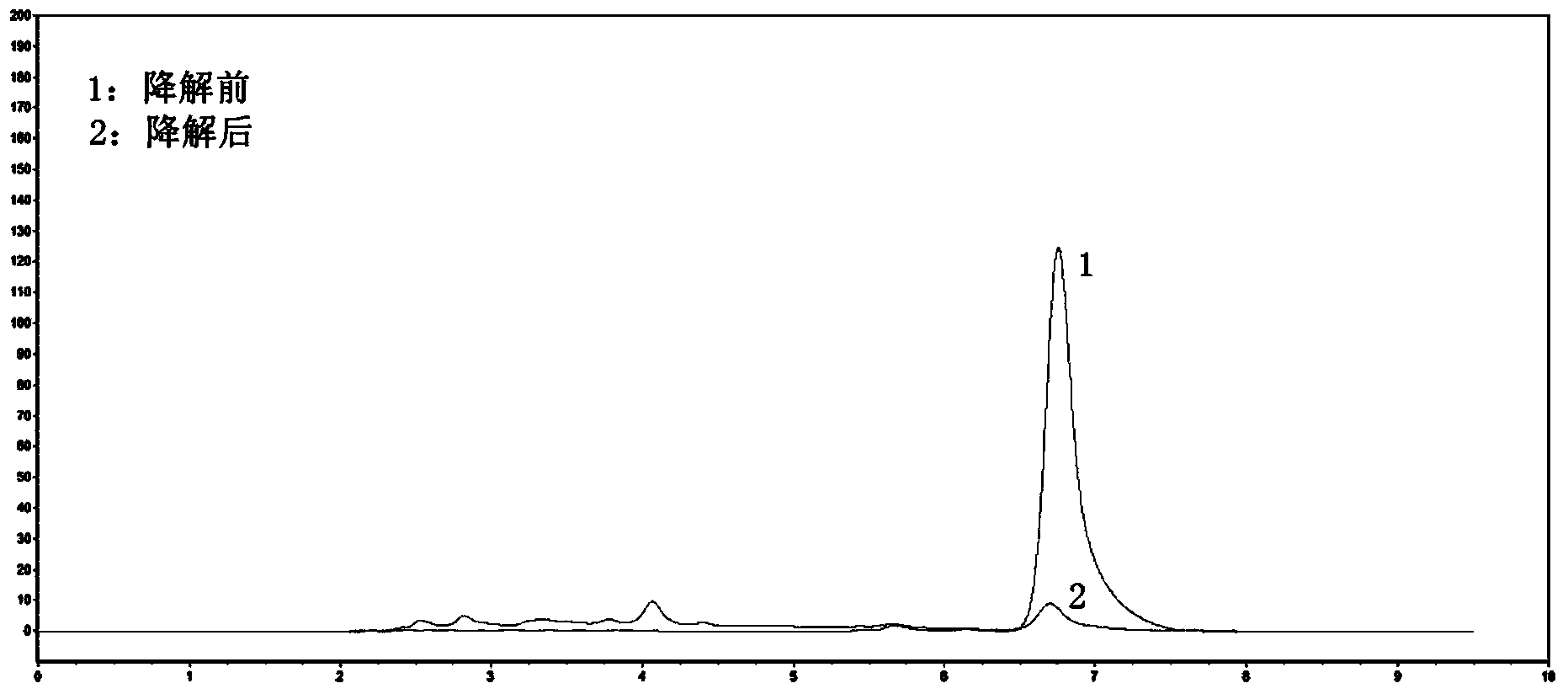
[0042] Select the strains preserved by our research group, inoculate them into the improved inorganic salt medium, add methyl parathion with a final concentration of 100ppm, cultivate them at 30°C and 200rpm for 1-2 weeks, pass them three times, and use TLC Analysis and high performance liquid chromatography to identify the degradation effect of strains on methyl parathion.
[0043] The composition of the improved inorganic salt medium is: 1g / L NaCl, 1g / L NH 4 NO 3 , 1.5g / L K 2 HPO 4 , 0.5g / L KH 2 PO 4 , 0.2g / L MgSO 4 ·7H 2 O, 1g / L yeast extract.
[0044] The cultured bacteria solution was extracted three times with chloroform-ether (1:1, v / v). After evaporating the extractant at room temperature, it was dissolved in methanol and used for thin-layer chromatography and high-performance liquid chromatography analysis.
[0045] The thin-layer chromatography developing solvent is n-hexane:chloroform...
Embodiment 2
[0055] Preparation and activity screening of site-directed saturation mutants of PoOPH
[0056] The saturation mutants at positions 250 and 263 of the PoOPH gene were constructed respectively.
[0057] The PCR primers were designed as follows:
[0058] H250X-F: 5′-ATGGGGCGACATTCTG NNK AACCACGCCGTGCAG-3′
[0059] H250X-R: 5′-CTGCACGGCGTGGTT MNN CAGAATGTCGCCCCAT-3′
[0060] I263X-F: 5′-CCAAGCCTGAAGTGGTC NNK GAGTTCGATGTCGACA-3′
[0061] I263X-R: 5′-TGTCGACATCGAACTC MNN GACCACTTCAGGCTTGG-3′
[0062] The underlined part is the degenerate codon of the mutation site. Wherein, N represents any one of the four bases A, C, G, and T, M represents any one of the two bases T and G, and K represents any one of the two bases A and C.
[0063] Using the recombinant expression plasmid containing the pooph gene obtained in Example 1 as a template, PCR site-directed mutagenesis was performed on the whole plasmid.
[0064] The above-mentioned complete plasmid PCR site-directed mutag...
Embodiment 3
[0073] Purification of PoOPH and its mutants and determination of pure enzyme activity
[0074] Use Ni affinity chromatography column (GE company HisTrap TM FF column, 5mL) to purify PoOPH and its mutant proteins PoOPH(H250I), PoOPH(I263W) and PoOPH(H250I / I263W), the specific method is as follows:
[0075] (1) Equilibrate the Ni chromatography column with Tris-HCl buffer (20mM, pH8.0, containing 0.5M NaCl and 20mM imidazole) of 5 times the bed volume;
[0076] (2) The crude enzyme liquid obtained in Example 2 is passed through the Ni chromatography column with a flow rate of 1ml / min;
[0077] (3) Use 5 times the bed volume of 20mM pH8.0 Tris-HCl buffer (containing 0.5M NaCl and 20mM imidazole) to elute non-specifically bound foreign proteins;
[0078] (4) Elute the specifically bound target protein with 5 times the bed volume of 20 mM pH 8.0 Tris-HCl buffer (containing 0.5 M NaCl and 0.5 M imidazole).
[0079] The purified proteins PoOPH, PoOPH(H250I), PoOPH(I263W) and PoO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com