Benzamide or acylhydrazone derivative containing 5-trifluoromethyl-4-pyrazole amide structure, and application of derivative
A technology of trifluoromethyl and benzoyl hydrazone, applied in the application, biocide, animal repellent and other directions, can solve problems such as control difficulties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
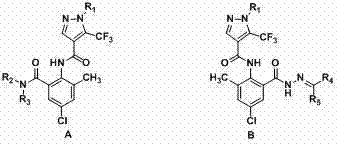
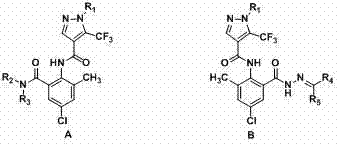
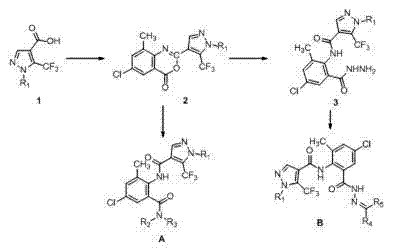
[0028] Example 1: N -(4-Chloro-2-methyl-6-(methylcarbamoyl)phenyl)-1-(3-chloropyridin-2-yl))-5-(trifluoromethyl)-1H-pyridine Preparation of azole-4-carboxamide (A2)
[0029] 1) 6-chloro-2-(1-(3-chloro-2-pyridyl)-5-trifluoromethyl-1H-4-pyrazolyl)-8-methyl-4H-benzo[d] Preparation of [1,3]oxazin-4-one (2)
[0030] In a 100 mL three-neck flask with a condenser tube and a thermometer, add pyridine (34.3 mmol), compound 1 (17.2 mmol) and methanesulfonyl chloride (51.4 mmol), stir at room temperature for 5 min, and then add 2-amino -5-Chloro-3-methylbenzoic acid (68.6 mmol), reacted at room temperature for 4 hours, then added 30 mL of water to the system, a large amount of solids precipitated, filtered with suction, washed the filter cake with water, and dried to obtain Intermediate 2, a yellow solid , yield 89.6%, melting point 179~181 o c. 1 H NMR (DMSO-d 6 ): δ 8.68 (d, J = 5.2, 1H, pyridine H), 8.66 (s, 1H, pyrzole H), 8.41 (d, J = 8.0 Hz, 1H, pyridine H), 7.96 (s, 1...
Embodiment 2
[0033] Example 2: N -(4-Chloro-2-(2-((dimethylamine)methylene)acylhydrazone)-6-tolyl)-1-(3-chloropyridin-2-yl)-5-(trifluoromethane base)-1H-pyrazole-4-carboxamide (B2) preparation
[0034] 1) N -(4-Chloro-2-(hydrazide)-6-methylphenyl)-1-(3-chloropyridin-2-yl)-5-(trifluoromethyl)-1H-pyrazole-4-methyl Preparation of amides (3)
[0035] In a 50 mL three-necked flask, add 80% hydrazine hydrate solution (13.6 mmol), 2 (6.8 mmol) and THF (15 mL), and react at room temperature for 1 hour. A large amount of solids precipitated in the system, which was suction filtered, and the filter cake was washed with anhydrous After washing with ethanol and drying, intermediate 3 was obtained, a white solid with a yield of 93.0% and a melting point of 158-160 o c. 1 H NMR (DMSO-d 6 ): δ 10.22 (s, 1H, NH), 9.64 (s, 1H, NH), 8.66 (d, J = 4.6 Hz, 1H, pyridine H), 8.44 (s, 1H, pyrazole H), 8.39 (d, J = 8.0 Hz, 1H, pyridine H), 7.82-7.79 (m, 1H, pyridine H), 7.52 (s, 1H, Benzene H), 7.54 (s, ...
Embodiment 3
[0042] Example 3: Biological Activity Test on Plutella xylostella
[0043] Determination by dipping leaf feeding method[ pesticide , 1996 , 35(6): 37-39], immerse fresh cabbage leaves in the liquid medicine for 10 s and then treat the test insects, and the test insects should be starved for 5-6 hours before treatment. Each concentration treatment was repeated 3 times, and 10 test insects were repeated for each repetition. The immersion solvent was used as the control. The treated test insects were placed in a moisturizing petri dish with filter paper and kept in an insect breeding room at a constant temperature. They died within 72 hours after inspection. number of worms and calculate the mortality rate of the worm population. As shown in table 2.
[0044] Calculate the mortality rate and the adjusted mortality rate:
[0045] (1)
[0046] In the formula: P1—mortality rate; K—the number of dead insects; N—the total number of insect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com