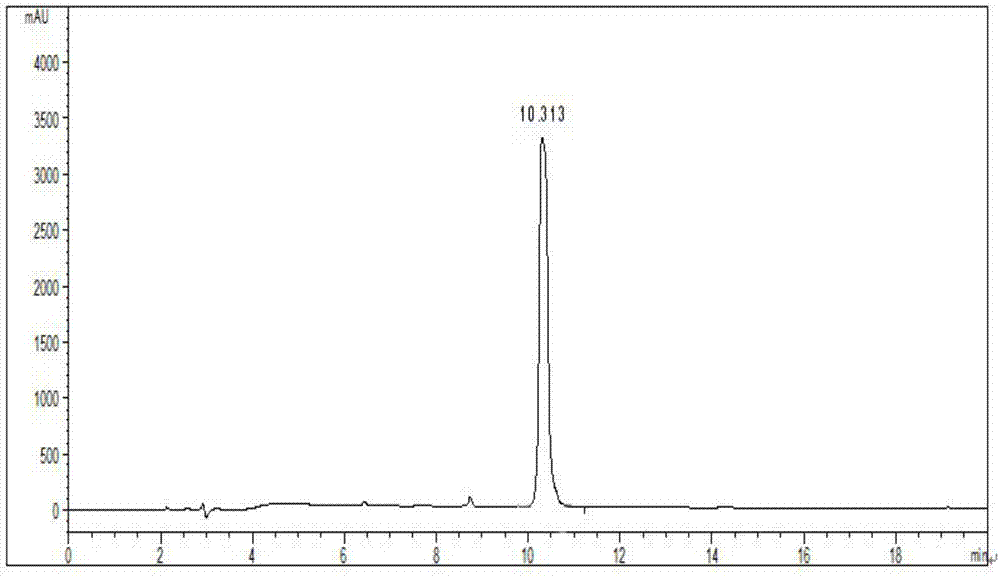
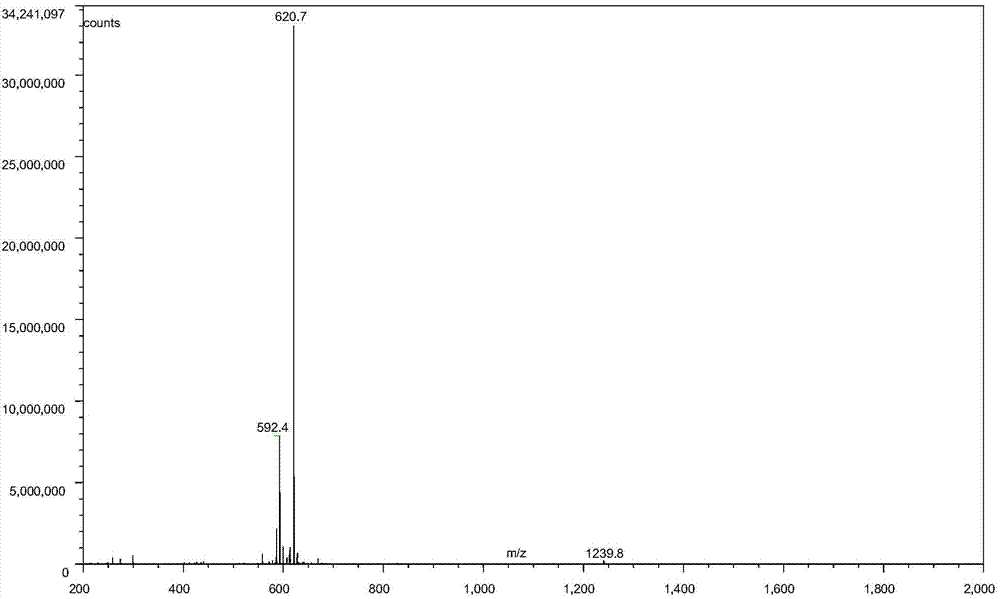
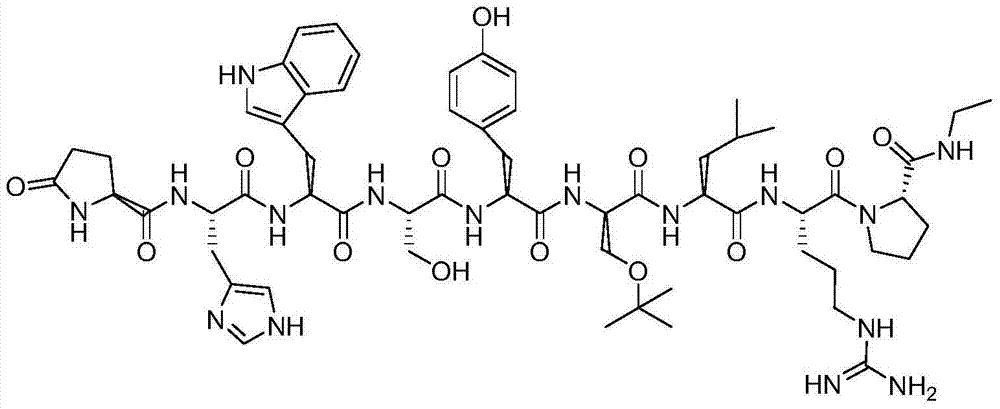
Buserelin preparation method
A technology of resin and conditions, applied in the field of preparation of buserelin, can solve the problems of difficult realization of strategies, complex protection strategies, expensive Rink and Sieber resins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: preparation buserelin
[0061] The implementation steps of this embodiment are as follows:
[0062] A. Using solid-phase synthesis, Fmoc-Pro with a degree of substitution of 0.4mmol / g was obtained from Fmoc-Pro-OH and a CTC resin with a degree of substitution of 0.6mmol / g in the presence of N,N-diisopropylethylamine -CTC resin; wherein the molar ratio of CTC resin to N,N-diisopropylethylamine is 1:3;
[0063] B. Treat the Fmoc-Pro-CTC resin obtained in step A with a solution of 10% piperidine in N,N-dimethylformamide by volume to remove Fmoc in the protected amino acid. After removing Fmoc, in the same manner as step A, according to the peptide sequence Pyr-His(Trt)-Trp-Ser-Tyr(Bzl)-D-Ser(tBu)-Leu-Arg(NO 2 )-Pro-OH, using coupling agent DIC / HOBt and organic base N,N-diisopropylethylamine to gradually couple and protect amino acids to obtain buserelin-CTC resin;
[0064] C. In a solution of 0.5% trifluoroacetic acid in dichloromethane by volume, the buse...
Embodiment 2
[0070] Embodiment 2: preparation buserelin
[0071] The implementation steps of this embodiment are as follows:
[0072] A. Using solid-phase synthesis, Fmoc-Pro with a degree of substitution of 0.5 mmol / g was obtained from Fmoc-Pro-OH and a CTC resin with a degree of substitution of 0.7 mmol / g in the presence of N,N-diisopropylethylamine. -CTC resin; wherein the molar ratio of CTC resin to N,N-diisopropylethylamine is 1:4;
[0073] B. Treat the Fmoc-Pro-CTC resin obtained in step A with a solution of 15% piperidine in N,N-dimethylformamide by volume to remove Fmoc in the protected amino acid. After removing Fmoc, in the same manner as step A, according to the peptide sequence Pyr-His(Trt)-Trp-Ser-Tyr(Bzl)-D-Ser(tBu)-Leu-Arg(NO 2 )-Pro-OH, using coupling agent PyBOP / HOBt and organic base N,N-diisopropylethylamine to gradually couple and protect amino acids to obtain buserelin-CTC resin;
[0074] C. In a solution of 1.0% trifluoroacetic acid in dichloromethane by volume, the...
Embodiment 3
[0080] Embodiment 3: preparation buserelin
[0081] The implementation steps of this embodiment are as follows:
[0082] A. Using solid-phase synthesis, Fmoc-Pro-OH with a substitution degree of 0.8mmol / g was obtained from Fmoc-Pro-OH and a CTC resin with a substitution degree of 0.8mmol / g in the presence of N,N-diisopropylethylamine. Fmoc-Pro with a substitution degree of 0.6mmol / g -CTC resin; wherein the molar ratio of CTC resin to N,N-diisopropylethylamine is 1:5;
[0083] B. Treat the Fmoc-Pro-CTC resin obtained in step A with a solution of 20% piperidine in N,N-dimethylformamide by volume to remove Fmoc in the protected amino acid. After removing Fmoc, in the same manner as step A, according to the peptide sequence Pyr-His(Trt)-Trp-Ser-Tyr(Bzl)-D-Ser(tBu)-Leu-Arg(NO 2 )-Pro-OH, using coupling agent TBTU / HOBt and organic base N,N-diisopropylethylamine to gradually couple and protect amino acids to obtain buserelin-CTC resin;
[0084] C. In a solution of 1.5% trifluoroac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com