Use of o-carbonyl oxime compound and preparation method thereof
A compound, carbonyl oxime technology, applied in the detection of nerve agents, preparation of o-carbonyl oxime compounds, detection of nerve agents, especially G-type nerve agents, specific identification of G-type and V-type nerve agents, G-type Nerve agent field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
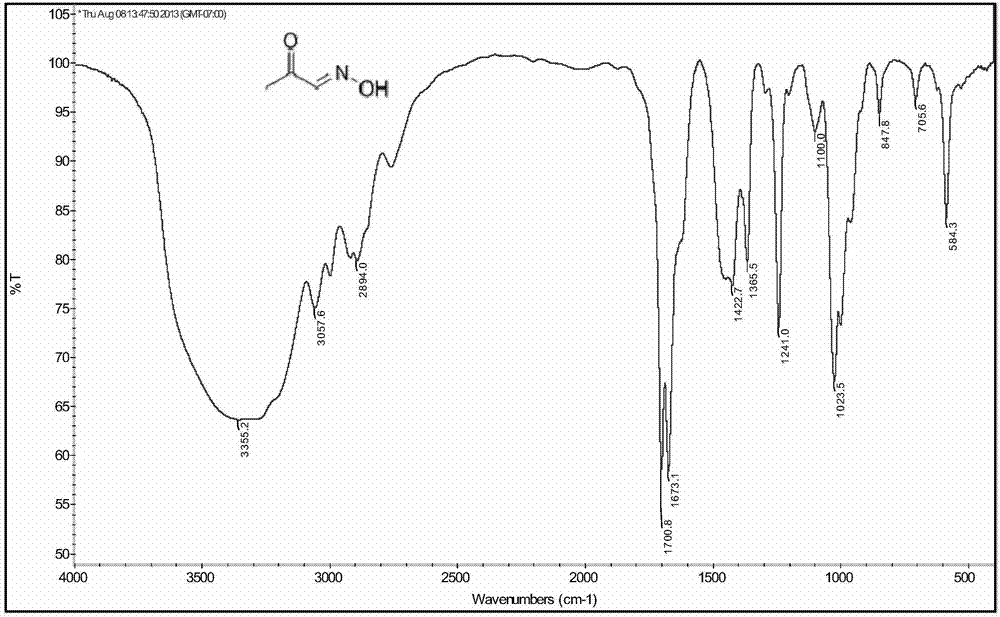
[0086] 2g (30mmol) methylglyoxal aqueous solution, 2g (28.5mmol) hydroxylamine hydrochloride, 0.2g (1.9mmol) Na 2 CO 3 Mix homogeneously with 4mL THF in a 100mL three-necked round-bottomed flask, and react with magnetic stirring at room temperature. During the reaction, use thin-layer chromatography (TLC) to monitor the raw material methylglyoxal (developing agent is n-hexane / ethyl acetate=10: 1, v / v), after about 3 hours of reaction, TLC showed that the raw material point was close to disappear, stop stirring, pour the reaction solution into a 250mL beaker, extract three times with THF, discard the aqueous phase, combine the organic phase, and use anhydrous Na 2 SO 4 The organic phase was dried overnight, and the solvent was removed by rotary evaporation to obtain a brown oily liquid, which was further purified by silica gel column chromatography (300 mesh, eluent: ethyl acetate:dichloromethane=1:1) to obtain a light yellow oily liquid, acetone aldoxime Its purity is high...
Embodiment 2
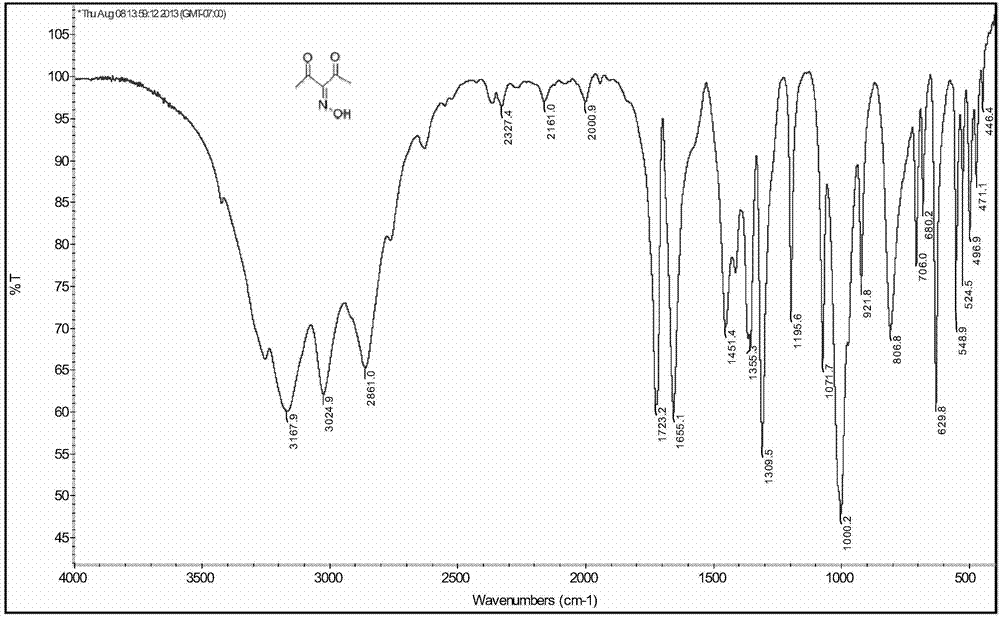
[0088] Mix 10g (100mmol) acetylacetone, 30mL acetic acid and 10mL ultrapure water in a 250mL three-necked round-bottomed flask evenly, place a magnetic stirrer and a thermometer in the three-necked flask, under nitrogen protection and ice bath conditions, stir for 10min, and then 8g (160mmol) NaNO 2 Dissolve in 10mL of deionized water, add dropwise to the acetic acid solution of acetylacetone with a constant pressure liquid adder, control the drop rate, and keep the reaction temperature at 8-10°C. After the dropwise addition is completed, remove the ice bath and re- The reaction was carried out for 1 h, and the raw materials and products were monitored by GC-MS. After stopping the reaction, the organic phase was extracted 3 times with anhydrous ether, and saturated NaHCO 3 After the solution was washed to neutrality, the organic phase was washed twice with distilled water, and the water phase was discarded after standing for stratification. 2 SO 4 After drying, diethyl ethe...
Embodiment 3
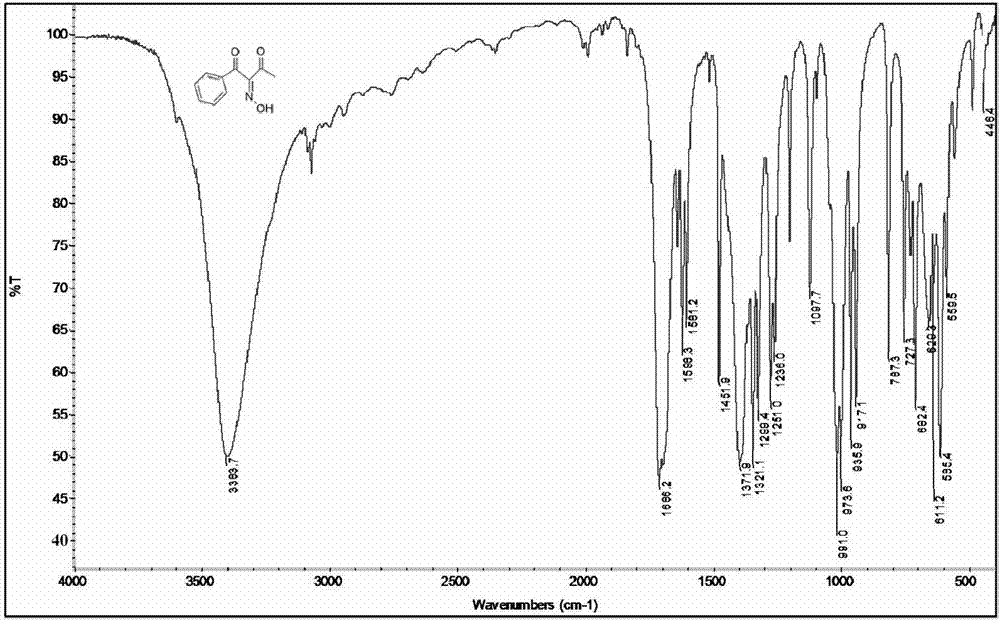
[0090] Mix 2g (12.3mmol) of 1-phenyl-1,3-butanedione, 15mL of acetic acid and 10mL of ultrapure water in a 100mL three-necked round-bottomed flask, and place a magnetic stirrer, a thermometer in the three-necked flask, and nitrogen protection And under ice bath conditions, after stirring for 10min, 0.93g (13.5mmol) NaNO 2 Dissolve in 10mL deionized water, add dropwise to the acetic acid solution of 1-phenyl-1,3-butanedione with a constant pressure dispenser, pay attention to control the rate of addition, keep the reaction temperature at 8-10°C, wait for the dropwise After the addition was complete, the ice bath was removed, and the reaction was carried out at room temperature for another 1 h, and the raw materials and products were monitored by GC-MS. After stopping the reaction, the organic phase was extracted 3 times with anhydrous ether, and saturated NaHCO 3 After the solution was washed to neutrality, the organic phase was washed twice with distilled water, and the water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com